Percutaneous coronary intervention (PCI) has evolved since its inception more than 40 years ago. There have been major advances in technology, with adaptations made across all facets of the procedure, from stent engineering to adjunctive physiology and intracoronary imaging. Despite this progress, the mainstay for all PCI procedures remains fluoroscopic X-ray imaging guidance, with manual manipulation of guidewires, balloons, stents and other devices. This has meant there has been little change in the occupational hazards for operators and catheterisation laboratory staff. Furthermore, although the anatomical complexity of percutaneous revascularisation has increased, there remains an innate degree of variability seen with human operative methods.
Medical robots are gaining widespread use in surgery because of high precision, speed, reproducibility, greater access to areas under operation and machine endurance, all features that are prone to the variability of human error.1–3 The use of robotic systems has expanded to incorporate the field of PCI. Despite accumulating evidence that supports the feasibility and safety of robot-assisted PCI (R-PCI), these procedures are only performed in a limited number of centres worldwide.4
Although the interventional cardiology community has a heightened awareness of the many potential hazards of working in the catheterisation laboratory, adoption of R-PCI has been slow, with concerns around learning curves, costs and adaptability in contemporary practice.5 This review outlines and summarises the current position, limitations and future potential of R-PCI.
What is Robotic Percutaneous Coronary Intervention?
An R-PCI system enables control of coronary guidewires and intracoronary devices, such as balloons and stents, during PCI from a protected control cockpit. The CorPath 200 (Corindus Vascular Robotics) was the first incarnation of an R-PCI system and was used in the initial feasibility trials. This system has been further improved upon, with the CorPath GRX (Corindus Vascular Robotics) being the current iteration.
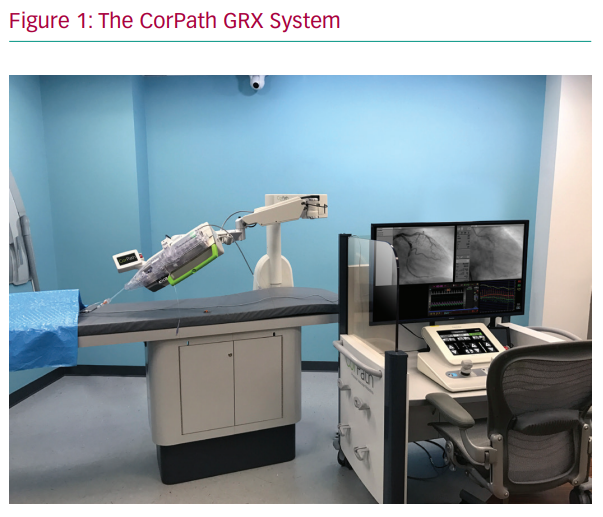
The CorPath GRX system is composed of two subunits: a bedside unit and the remote physician workspace (Figure 1). The bedside unit consists of the articulated arm, the robotic drive and a single-use cassette in which devices, including wires, balloons and stents, are loaded (by a member of the catheterisation laboratory team who remains within proximity of the patient). The remote workspace consists of an interventional cockpit, which is surrounded by a radiation shield and houses the control console, angiographic and haemodynamic monitors and the X-ray foot pedal.
During the procedure, the interventional cardiologist can sit comfortably within the shielded environment, almost completely eliminating radiation exposure, without needing to wear lead aprons. The operator may choose to have the cockpit ‘sterilised’, and thereby remain in a sterile gown throughout the procedure, or to perform the PCI without a sterile gown. The remote workspace can also be taken to the control room to completely eliminate radiation exposure. The latter facilitates removal of the operator’s lead garments during PCI. The system allows the operator to control and manipulate guidewires, balloon and stents using a set of joysticks and touch screens while fluoroscopy provides image guidance.6 Axial and rotational motion are achieved by a mechanical transmission module. The balloon or stent can be guided both in a continuous motion using the joystick and in discrete, highly sensitive small steps using the touch screen. Axial motion is achieved by the motored roller pair. If the device meets resistance and the motored rollers slide, the motion-sensing rollers report malfunction and the system halts.7
Potential Advantage for the Patient
Increased Procedural Accuracy
The main aim of using robotic systems in interventional cardiology is to provide increased procedural precision and improve efficiency in clinical care. Although the advent of various intracoronary imaging techniques (e.g. intravascular ultrasound [IVUS] and optical coherence tomography [OCT]) has undoubtedly increased the accuracy and precision of PCI procedures, the adoption of these techniques, in routine PCI procedures remains relatively low. The 2019 British Cardiovascular Intervention Society audit data indicate that only 13.2% of all PCI procedures (n=100,294) used either IVUS or OCT.8 There are numerous reasons for this, including fiscal ramifications surrounding reimbursement, the perception of increased procedural times and a reduction in catheter laboratory efficiency when these technologies are used routinely. Therefore, angiography-guided PCI continues to be the mainstay in contemporary PCI practice.
Following a PCI procedure, a major modifiable risk factor for further target vessel revascularisation is accurate stent selection during the index procedure, which is primarily influenced by operator experience and procedural technique. In a US multicentre observational registry that included >1,500 patients, incomplete coverage of the entire length of the coronary lesion was observed in 46.5% of cases, with incomplete lesion coverage (longitudinal geographic miss [LGM]) being associated with higher rates of target vessel revascularisation at 1 year, independent of clinical or anatomical risk factors.9 Furthermore, in a recent analysis of the accuracy of visual angiographic lesion assessment by interventional cardiologists, lesion length was underestimated by 51% and overestimated by 19%, highlighting the variance with the current angiography-guided reference standard.10
The use of visual angiographic assessment has specific limitations, particularly in stent length selection, where 2D angiographic imaging, most notably in curved and tortuous vessels, leads to foreshortening, which affects accurate measurement of length. When using R-PCI, a special measurement feature can be used, measuring the real length unrelated to the angiographic view and possible foreshortening. This is achieved by using the actual intravascular device and taking into account the distal and proximal edges of the artery segment: the balloon markers are advanced to the distal and proximal edges of the lesion of interest. The distal edge is marked as ‘0’ on the touch screen display. By withdrawing the marker to the proximal edge of the lesion, the distance travelled by the marker can be measured to provide lesion length. The R-PCI system can make submillimetre measurements, improving accuracy compared with the visual estimates currently used.
In a retrospective, propensity-matched cohort analysis, Bezerra et al. demonstrated that the incidence of LGM was greater in those treated with conventional PCI compared with R-PCI (43.1% versus 2.2%, respectively; p<0.0001).11 Subsequent data on stent length selection and the consequent health economics of more accurate device selection suggest that the use of R-PCI reduces variability in device selection, with a reduction in the use of extra stents by approximately 8%, and therefore reductions in both procedural cost and the risk of LGM.12
Potential Advantages for the Operator
Reduced Radiation Exposure
Although there is the potential of increased precision when using robotic assistance during PCI, the key advantage lies in the reduction of radiation exposure and orthopaedic risk to the operator and potentially other catheter laboratory personnel.13 Conventional PCI is performed while standing beside the patient, in close proximity to the ionising radiation source, which necessitates the use of heavy lead aprons for protection. Numerous occupational hazards, including orthopaedic complications related to the use of lead aprons and radiation-related complications, such as cataracts and, more seriously, malignancies, have been identified.14–17 Interventional cardiologists are reported to have the highest radiation exposure among health professionals, with an exposure per person per year that is 2- to 10-fold higher than that of diagnostic radiologists. The calculated cumulative dose after 30 years of working is in the range 50–200 mSv, with a projected professional lifetime attributable excess cancer risk in the order of 1 in 100.18
Although it is difficult to prove occupational radiation exposure to increased cancer risk, there is evidence that mandates caution. The Brain Radiation Exposure and Attenuation During Invasive Cardiology Procedures (BRAIN) study confirmed that radiation exposure to the cranium is higher on the left side during interventional cardiology procedures.19 The potential that this has a causal link to the development of brain tumours was alluded to in a study of physicians diagnosed with brain tumours, 85% of which were left sided in origin, with the majority of physicians diagnosed being interventional cardiologists.20
Furthermore, the clinically appropriate increase in the adoption of procedures undertaken via a radial artery approach means that interventional cardiologists are exposed to small but significantly higher doses of ionising radiation.21 More broadly, Venneri et al. reported that the cumulative exposure dose among catheter laboratory personnel over time was associated with an increased risk of malignancy.16 Although a number of safety precautions, including collimation, the use of dose reduction software and operator education, all significantly limit radiation exposure, the long-term adverse risk of exposure cannot be completely ameliorated.14,17
However, the advent and use of robots to assist with PCI have resulted in a marked reduction in operator radiation exposure. The Percutaneous Robotically Enhanced Coronary Intervention (PRECISE) study was the first demonstration of the safety and feasibility of R-PCI in a non-randomised multicentre registry of 164 patients undergoing R-PCI.4 Importantly, radiation exposure for the primary operator was 95.2% lower than the levels found at the traditional table position.4 This reduction in radiation is in concert with the procedure being performed with the operator seated and without any lead apron, which also mitigates some of the orthopaedic hazards facing PCI operators.
Improved Ergonomics
One of the major advances with the advent of robotic technology is the potential of more ergonomic working in the catheter laboratory. PCI complexity has steadily increased with other technological advances, leading to interventional cardiologists spending increasing periods of time in lead aprons, which has a significant impact on the musculoskeletal system. A survey of interventional operators highlighted that 50% of respondents reported at least one occupational orthopaedic injury; these were commonly cervical and lumbar injuries, and were strongly correlated with both case load and advancing operator age.22
Not needing the heavy lead personal protective equipment and the ability to remotely control the procedure while in a seated position mean that R-PCI has the potential to minimise the risk of the long-term sequelae of current PCI working. However, the current systems do not allow for complete automation. The traditional manual method is still needed to obtain arterial access, perform diagnostic coronary angiography and intubate the guiding catheter. Once the guiding catheter is engaged, operators can remove the lead aprons and position themselves in the interventional cockpit.
Evidence Base Supporting Robotic-Assisted Percutaneous Coronary Intervention
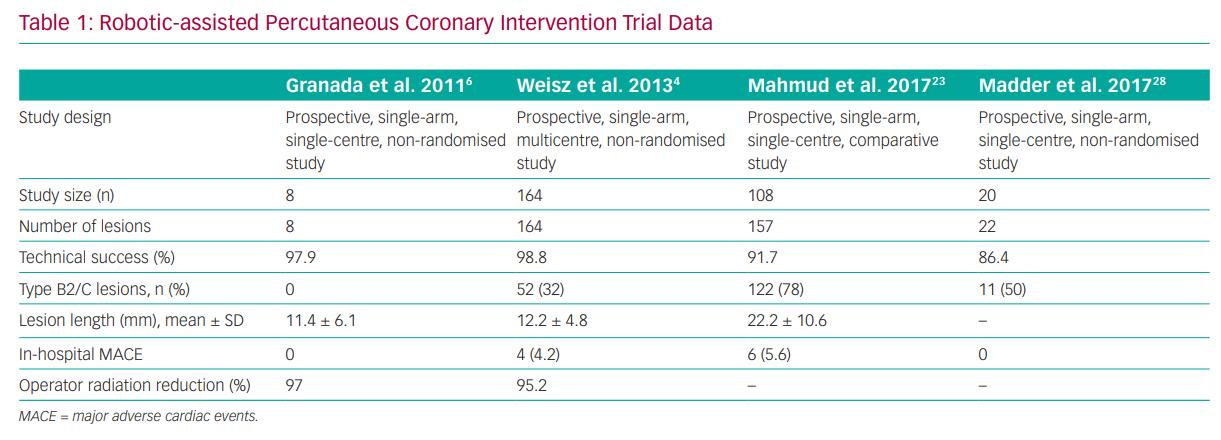
The pivotal assessment of R-PCI safety was seen in the PRECISE study, which included 164 patients with at least 50% diameter stenosis in vessels ranging from 2.5 to 4.0 mm in diameter that could be covered with a single stent.4 Key exclusion criteria were the presence of a previous stent within 5 mm of the planned stent deployment, planned atherectomy, intraluminal thrombus, severe tortuosity or calcification proximal to the lesion, ostial location, bifurcation lesion and unprotected left main lesions. Of the 164 patients, 112 (68.3%) had type A or B1 lesions, whereas the remainder had type B2 (18.9%) or type C (12.8%) lesions. Procedural success (without conversion to a conventional manual procedure) was achieved in 98.8% (n=162/164). There were no deaths, strokes, Q wave MIs or target lesion revascularisation after 30 days of follow-up.4
Although the findings of the PRECISE study confirmed both procedural safety and improved operator ergonomics and radiation safety, there are considerable concerns about the ability of R-PCI to perform revascularisation in more complex lesions and patient subsets. In the single-centre Complex Robotically Assisted Percutaneous Coronary Intervention (CORA-PCI) Study, consecutive patients undergoing complex R-PCI were compared to a manual PCI (M-PCI) control group.23 In that study, 315 patients (mean ± SD age 67.7 ± 11.8 years; 78% men) underwent 334 PCI procedures (108 R-PCIs: 157 lesions, 78.3% type B2/C; 226 M-PCIs: 336 lesions, 68.8% type B2/C). The technical success with R-PCI was 91.7%, with a 11.1% rate of manual assistance and a 7.4% rate of manual conversion and no difference in clinical success compared with M-PCI (99.1% versus 99.1%, respectively; p=1.00). However, procedure time was longer in the R-PCI than the M-PCI group (mean ± SD 44:30 ± 26:04 versus 36:34 ± 23:03 min; p=0.002), despite a similar fluoroscopy time (mean ± SD 18.2 ± 10.4 versus 19.2 ± 11.4 min, respectively; p=0.39).23 Although this was insightful in showing the potential of R-PCI in the treatment of more challenging lesion subsets, there were notable exclusions that were ineligible for R-PCI, including patients who required atherectomy, a planned two-stent strategy for bifurcation lesions and chronic total occlusions that required a hybrid approach (Table 1).
The current robotic system is limited to rapid exchange (monorail) devices only, meaning that rotational or orbital atherectomy, which require the use of specialised wires and an over-the-wire technique, are not possible. However, recently, case descriptions of the safe use of laser atherectomy, which can be performed using routine guidewires, as an alternative lesion modification device have been published.24 Furthermore, there have been descriptions of the use of R-PCI in multivessel coronary disease, saphenous venous graft disease, left main stem disease and in the setting of ST-elevation myocardial infarction.25,26 This suggests that a combination of developing operator technical expertise and continued iteration of the engineering of the robotic system could allow the envelope of R-PCI to expand further.
Current Limitations
The adoption of R-PCI has been slow for a number of reasons. Most importantly, there is a lack of robust clinical data, with no randomised clinical trials with the currently available systems. Most of the available data are based on small or medium-sized clinical registries of highly selected patients with relatively simple coronary lesions. There is a need for clinical evidence from large-scale randomised clinical trials showing improved radiation safety for the operators and non-inferior angiographic and clinical results across a broad spectrum of patient and lesion subsets.
The healthcare funding infrastructures and resource utilisation across many countries and systems mean that there is concern about the costs of installing and operating R-PCI systems. Increasingly many hospitals have multiple catheter laboratories, where there are numerous procedures being performed simultaneously. The current robotic systems can only be installed in a single room within the catheterisation laboratory environment. This will limit more widespread, systematic use, unless numerous systems are purchased, which clearly has considerable fiscal implications. In addition to clinical data about the utility of R-PCI, further data are required through well-designed health economic studies as to whether R-PCI systems confer an advantage if adopted on a more generic scale for healthcare systems. Moreover, with regard to resource utilisation, it is important to point out that R-PCI can be associated, particularly in the early phase of the learning curve, with prolonged procedural time compared with conventional manual PCI. This is seemingly overcome rapidly after a period of consistent use, but the nature of the initial phase of use needs to be considered when adopting the technology.
Technically, the initial CorPath 200 system had several limitations, which included the lack of haptic mechanical feedback, the inability to manipulate guiding catheters during complex cases and the inability to use over-the-wire equipment (e.g. microcatheters, rotational atherectomy) or to control more than one wire and balloon or stent. The subsequent version, the CorPath GRX, overcame some of these limitations. Importantly, the CorPath GRX allows for guide catheter control and manipulation. This is important for active guide support during intracoronary intervention, including challenging coronary anatomy.
Although much of the data obtained shows excellent technical success rates with R-PCI, albeit in narrow, highly selected groups, many interventional cardiologists feel that the lack of tactile sensation remains a limiting factor. The inability to detect variance in tactile feedback when using wires that have different mechanical properties (e.g. polymer coated, hydrophilic, varying lubricity) is important in complex cases, where the interaction between the wire, lesion and operator is key in understanding lesion morphology and subsequent technical success. Further development and advancement of haptics within the robotic system will allow for a more natural interaction between the wire and operator. Furthermore, the current iteration of the robotic system does not allow the remote use of intracoronary imaging. This limits the PCI to angiographic guidance only. However, as the system is improved upon and advanced, this may change to allow adjunctive imaging to be added to the portfolio of devices that could be used during R-PCI.
The issue of R-PCI being limited to less complex lesion subsets is due, in part, to the fact that current robotic systems do not support over-the-wire coronary interventions. Therefore, adjunctive tools and techniques, such as rotational and orbital atherectomy for calcium modification, the use of microcatheters and aspiration devices, cannot be used with R-PCI. In addition, the current systems do not support planned coronary bifurcation stenting with a two-stent approach. With advanced coronary interventions becoming more common, this limitation means that a major portion of the procedure needs to be performed manually.
Finally, R-PCI does not completely ameliorate scattered radiation risk. Although the interventional operator sits within a shielded environment protected from ionising radiation, other members of the team, technicians and fellows, are still required to stay within the radiation field during the procedure to inflate the balloon and stents, and therefore may be less motivated to adopt this new technology. Furthermore, robot-assisted systems do not currently offer the operator a means to decrease radiation exposure during diagnostic procedures.
Future Developments
The current robotic systems are in the relatively early stages of development compared with established modes of working and techniques in PCI practice, which have been developed and been iterated upon over the past 40 years. However, the robotic systems are continuously improving. Their scope for more accurate intervention and improved ergonomic working is evident already. Further technological advancements will further improve R-PCI and allow it to be adopted across a wider group of patients and lesion subsets.
A key potential advancement that robotic assistance could bring is in the field of ‘telerobotics’. This could allow robot-assisted PCI systems to treat patients who are in geographically distant locations. This could be invaluable for patients who otherwise could not be transported in time to a PCI-capable hospital, potentially reducing door-to-balloon times in those that are in remote locations. Contemporary communication systems have allowed for the use of telerobotics in the surgical arena, which is now in routine use.
A Canadian telerobotic surgical service was developed between a teaching hospital and rural hospital for the provision of a variety of advanced laparoscopic surgeries in their community patients.27 This early description of a telerobotic service showed the feasibility and safety of such a service with increasingly complex laparoscopic surgical operations, with no intraoperative complications or conversion to open operations.
The REMOTE-PCI study demonstrated the potential feasibility of such an approach.28 In that small study (n=20), the interventional cockpit of the robotic system was removed from the catheter laboratory with the patient in situ and placed behind the closed doors of an isolated room, with no direct visual or auditory contact with the patient or catheterisation laboratory team. Communication between the operators and the laboratory personnel occurred via telecommunication devices providing real-time audio and video connectivity, with a technical success rate of 86%.
To achieve robotic PCI with a remote operator location, additional data, including video displays similar to those used for telemedicine, would be needed to allow the operator to observe the patient and the procedure room environment. In addition, added controls would be needed on the console, such as camera controls, table and C-arm controls, dye injectors and, ideally, a microphone with headset so that the operator could communicate directly with those in the procedure room in real time. Although this off-site approach is promising, there will still be a need for a local experienced operator who would be able to address procedural complications. Patel et al. recently described the first ‘off-site’ robot-assisted PCI in a cohort of five patients, all of whom underwent successful, uncomplicated PCI procedures for Type A coronary lesions.29 This confirmed the feasibility of the concept in the presence of appropriate local cardiac catheterisation facilities and clinical support with reliable network connectivity.29
Finally, there is an increasing need for neurointervention in the treatment of cerebrovascular accidents, with a lack of sufficiently skilled operators to treat this critically unmet patient subset. This could be bridged by the use of PCI operators’ technical skillsets and telerobotics to provide remote care in populations in many parts of the world that have limited access to prompt neurointerventional treatments.
Conclusion
R-PCI is an emerging technology with significant potential for iterating upon current PCI methods. R-PCI is safe and feasible in a variety of lesion subsets, with clinical efficacy comparable to the conventional approach, possibly with increased procedural accuracy. In addition, R-PCI appears to provide operators protection from both radiation exposure and orthopaedic injuries.
The potential utility of telerobotic PCI systems to reduce costs and foster wider access to specialist coronary care by allowing interventional cardiologists to perform off-site procedures in remote locations would represent a major advancement in cardiac care. However, to reach their full potential, the next versions of robotic systems must address the limitations of the current generation of devices, which include a lack of compatibility with over-the-wire devices and the inability to manipulate multiple devices simultaneously to allow for more complex PCI cases to be completed without manual conversion. R-PCI represents a technique with great promise, and although improvements need to be made, greater adoption of the technique may perpetuate further improvements in technology and network-based care.