The timing of intervention in aortic stenosis (AS) is crucial. It is evident that severe AS is associated with poor survival when left untreated.1 Although current guidelines recommend aortic valve replacement (AVR) in patients with symptomatic severe AS or evidence of left ventricular dysfunction (left ventricular ejection fraction [LVEF] <50%), there is growing evidence that this treatment paradigm may lead to intervention after significant cardiac damage has occurred, with resultant poorer outcomes.2–4 Some patients with symptoms of decompensation (breathlessness, chest pain or syncope) only have moderate AS, yet have no other pathologies to which their symptoms can be attributed.
AVR may be considered in patients with moderate AS who are undergoing surgery for other indications.2,3 Current guidelines suggest that patients with moderate AS (defined as peak transaortic velocity of 3.0–3.9 m/s or mean gradient [MG] of 20–39 mmHg) should undergo surveillance yearly, and AVR deferred until the AS becomes severe. Transcatheter aortic valve replacement (TAVR) is currently not indicated in moderate disease. However, there are no established recommendations for the treatment of moderate AS with left ventricular systolic dysfunction, despite growing evidence of morbidity and mortality in this population.5 As with most guideline recommendations in valvular heart disease, there is a lack of randomised control trial data to support or refute earlier intervention in these patients.
The Biology of Aortic Stenosis
It is recognised that AS is not only a disease of the valve but also of the myocardium.6 Longstanding AS causes an increase in afterload, which leads to a myocardial remodelling response. This initial adaptive process eventually becomes maladaptive, and determines disease progression, symptoms and outcomes. The left ventricular response to AS is complex and consists of a combination of wall thickening, concentric remodelling and change in cavity size to maintain wall stress. In the later maladaptive stages, capillary rarefaction and cell death occur, as well as focal and diffuse myocardial fibrosis. How and when these processes develop is poorly understood, although sex differences appear particularly important.7 The afterload response conferred by the vasculature is important and varies between individuals. The prevalence of atherosclerosis and hypertension is also high in these patients, which can accelerate arterial stiffness.
AS – conventionally defined in binary terms (moderate or severe) – is better characterised by a number of relatively distinct phenotypes and how these relate to clinical courses/outcomes. Because conventional high gradient does not develop in many cases, pathological changes must be occurring at an earlier point in the disease process in some, if not all individuals. This is supported by the strong signal of symptom limitation at the moderate severity.
The Progression of Aortic Stenosis
The natural history of severe, symptomatic AS has been extensively documented.8–9 However, there is much less information on clinical outcomes in adults with moderate AS and the existing literature is heterogeneous. It is important to differentiate the anatomical progression of stenosis (‘how narrow?’) and the clinical progression with adverse outcome (‘how bad?’).
Anatomical Progression
There is a wide range in the progression rate, and it is unclear why some patients have a rapid disease progression and some do not. Haemodynamic AS progression is classically considered as constant and homogeneous (average increase in peak velocity by 0.3 m/s per year and an increase in MG by 7 mmHg per year) but is highly variable among individuals. As yet, no medical therapies have proven effective in reducing progression of AS or improving clinical outcomes.10
Clinical Progression
Earlier studies based on patient groups identified at cardiac catheterisation suggested a relatively benign prognosis in patients with moderate AS.11,12 Horstkotte et al. demonstrated a time interval between the first manifestation of moderate AS and progression to severe stenosis requiring surgery of 13.4 years.12
Later studies suggest that both clinical outcomes and progression to severe AS occur at a very high rate.13–20 Comparison of outcomes in patients with moderate AS is difficult because of the marked differences between studies (differences in symptom status, definition of stenosis severity and modality used to assess AS, study design, choice of end-points and definition of event-free survival). Nevertheless, moderate AS is associated with a substantial increase in mortality from both cardiac and non-cardiac causes. Furthermore, progression to severe disease can occur much more rapidly than previously expected.
The studies also identify characteristics (e.g. moderate or severe aortic valve calcification and concomitant coronary artery disease) that may put certain patients at risk for more rapid progression of AS.21 Data from the Heart Valve Clinic International Database registry showed that in patients with moderate AS at baseline, independent determinants of cardiovascular mortality were dyslipidaemia and peak velocity. There was also a trend for association between cardiovascular mortality and LVEF.22
Observational data from a large Australian registry sought to determine more definitively the prognostic impact of increasing severity of AS. Echocardiographic findings from 3,315 patients with moderate AS were linked to long-term mortality. The 5-year mortality was 56%, after adjusting for age, sex, left ventricular systolic or diastolic dysfunction and aortic regurgitation. The risk of dying in the longer term was similar to the risk in patients presenting with severe AS at baseline.5 In the population-based study by Livanainen et al., moderate AS was associated with an increased risk of all cause and cardiovascular mortality; however the independent predictive value was lost when left ventricular mass and systolic function were included in the analysis.14 The studies detailing clinical outcomes in patients with moderate AS are presented in Table 1.
It is also important to note that patients with moderate mixed aortic valve disease (AS and regurgitation) are at risk for all-cause mortality, and that AVR significantly reduced this risk, independent for aortic valve area (AVA), symptom status and after controlling for confounding variables.23
Aortic Stenosis and Left Ventricular Dysfunction
Left ventricular dysfunction has been reported in a quarter of patients with AS.24 The natural progression of AS comprises different phenotypic presentations involving patients who will develop left ventricular dysfunction and symptoms early, much before reaching the limit definition of severe AS. Both AS severity and left ventricular dysfunction are variables with definitions of ‘severe’ and ‘abnormal’ based on observational clinical studies.25,26 However, there is some evidence to suggest that patients with conventionally defined moderate AS and heart failure may benefit from AVR.27–29
The study by van Gils et al. showed that 61% of patients with moderate AS and left ventricular systolic dysfunction died, underwent AVR or were hospitalised for heart failure, at 4-year follow-up with male sex, New york Heart Association Class III or IV, and higher peak aortic velocities independently predicting events.26 A retrospective analysis from the Duke Echocardiographic database reported a mortality benefit associated with AVR (with or without concomitant coronary artery bypass grafting) for moderate AS and left ventricular systolic dysfunction (LVSD), compared with medical management.28 These findings differ from those of Fougeres et al., who found that in patients with pseudo-severe (moderate) AS, there was no difference in the survival rate compared to controls with LVSD and no valve disease.29
Data from Hayward et al. identified 169 patients with moderate AS and left ventricular impairment (AVA 1–1.5 cm2 and LVEF <50%) and sought to identify determinants of outcomes. The primary endpoint was all cause mortality with a median follow-up period of 22.9 months. Patients with abnormal global longitudinal strain (GLS) had significantly worse survival and the only independent predictor of outcome was GLS.30 A recently published study by Ito et al. showed that in patients with moderate AS, those with decreased LVEF and/or indexed stroke volume were at increased risk of mortality.31
Evidence for Benefit from Earlier Intervention
In patients with symptomatic AS (AVA <1 cm2) who had a low gradient and normal flow (NFLG), intervention was associated with reduced cardiac-related mortality, as opposed to conservative treatment.32 Long-term data are equivocal. These findings are similar to another study that demonstrated a similar long-term mortality when comparing NFLG patients with patients with moderate AS.33 In contrast, another study found a similar survival in patients undergoing early AVR compared with watchful waiting .34
Moon et al. reported that among 255 patients with moderate AS and concomitant LVSD, 47.5% of patients died and 18.8% received AVR during a median of 1.8 years of follow-up. The incidence of all cause death was significantly lower in the early AVR group compared with the medical treatment group. The LVEF also improved to >50% in 59.5% of the early AVR group. Limitations include the single-centre nature and small patient numbers.35
The investigators of the TAVR-UNLOAD trial aim to provide further insight into moderate AS with left ventricular dysfunction. Three hundred patients will be randomised into two arms: TAVR combined with optimised heart failure therapy versus optimal heart failure therapy alone. The primary endpoint will be a composite of all-cause death, disabling stroke, heart failure hospitalisations, symptomatic aortic valve disease or non-disabling stroke.36
Hypertension and concurrent coronary artery disease are common in patients with AS. Hypertension may also be a risk factor for AS and adds to the total pressure load on the left ventricle. Medical therapy for hypertension follows standard guidelines, starting at a low dose and titrating upwards as needed to achieve blood pressure control. All patients should be screened and treated for hypercholesterolaemia.3
The current data have important clinical implications. Whether they support AVR in patients with moderate AS before progression to severe is open to debate. However, it is important to question the conventional guidelines of expectantly managing these patients, suggesting that they require much closer follow-up and more aggressive management than the present guidelines indicate.
The Role of Multi-modality Imaging
Echocardiography
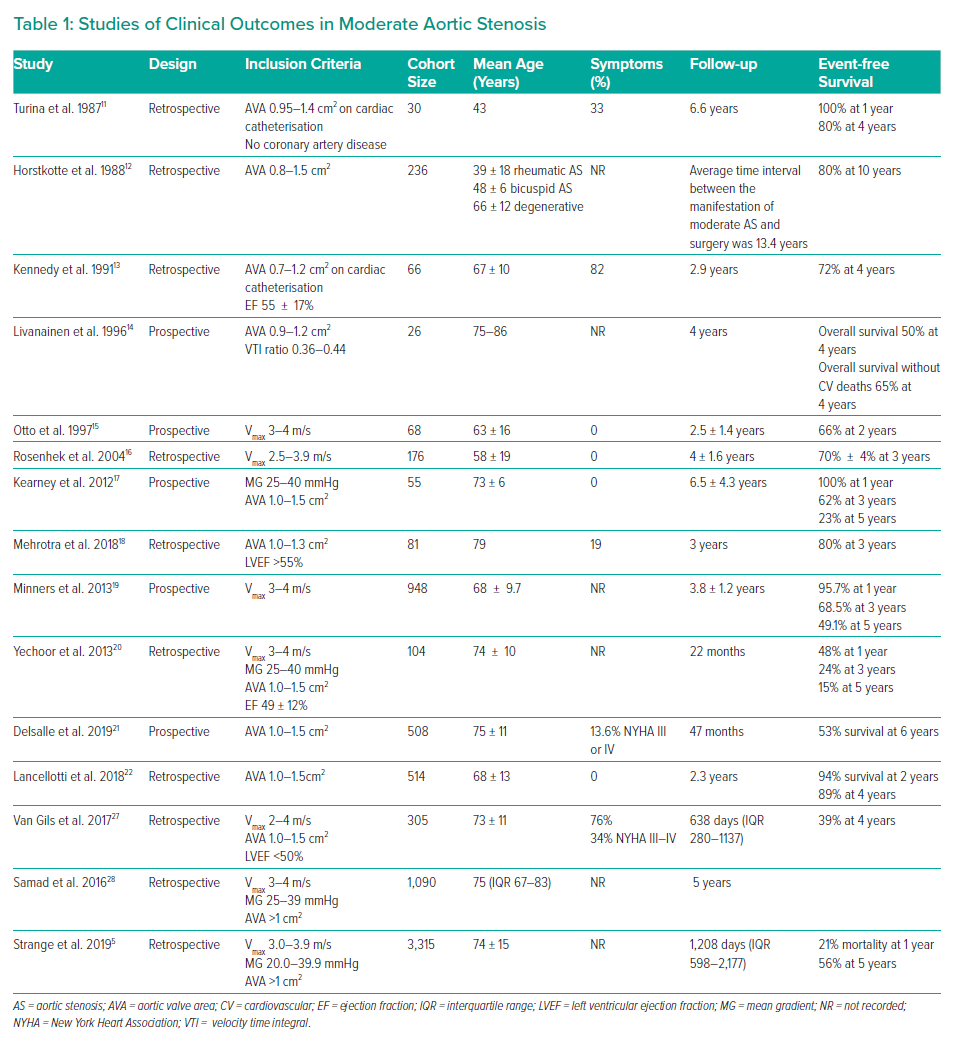
Echocardiographic evaluation starts with a visual assessment of the structure, calcification and mobility of the valve. AS severity is conventionally graded by peak velocity, MG and AVA (Figure 1). The classification of AS severity is not always straightforward, and echocardiographic findings are discordant in one in three patients.37 A distinction needs to be made between those with low flow (<35 ml/m2), and those with normal flow, in whom measurement inaccuracy or the discordance between a MG of 40 mmHg and a valve area of 1.0 cm2 are the more likely explanations. Additional investigations may be required. In patients with a reduced LVEF, low gradient and low AVA, dobutamine stress echocardiography may help in differentiating patients with true severe AS from pseudo-severe (moderate) AS.38
A decrease in flow may be because of an increase in global left ventricular afterload not only as a result of the valvular stenosis but also from a decrease in systemic arterial compliance and/or increased vascular resistance.39 Assessment of the global left ventricular haemodynamic load by measuring the valvuloarterial impedance (Zva) has been shown to be superior to the standard measures of AS severity in predicting left ventricular dysfunction and clinical outcomes.40,41 Hachicha et al. have also demonstrated that Zva is a strong independent predictor of clinical outcome in patients with at least moderate AS.41 The left ventricle faces a double load; valvular and arterial, particularly in hypertension, which frequently co-exists in patients with AS. A high Zva may explain why a patient with moderate AS is symptomatic. It is currently unclear whether these patients should undergo aortic valve intervention in the presence of symptoms, even after aggressive blood pressure control.
Assessment of the Left Ventricle
There is evidence to suggest that adaptive remodelling becomes maladaptive with increasing left ventricular hypertrophy and consequent myocardial fibrosis in patients with AS. Inappropriately high left ventricular mass (iLVM) is associated with increased mortality, AVR or hospital admission.42 iLVM is also common in asymptomatic mild-to-moderate AS and is associated with reduced left ventricular contractility.43
Left ventricular impairment is a strong adverse prognostic marker and LVEF <50% even in asymptomatic patients is a Class I indication for AVR. However, LVEF may remain normal until later in the course of the disease. Systolic long axis function may be impaired, even in the presence of a normal ejection fraction, in patients with AS (Figure 1).44
Asymptomatic patients with moderate AS have subclinical impaired systolic left ventricular function as measured by peak systolic tissue velocity (S’) and strain, compared to controls (S’ 4.1 ± 1.0 versus 4.8 ± 1.1 cm/s; p<0.01 and strain −16.6 ± 2.7 versus −17.9± 2%; p<0.05). Septal E’ (early diastolic mitral annular velocity) was decreased and septal E/E’ was increased in the AS group compared with the controls.45 Patients with AS may also develop symptoms because of progressive diastolic dysfunction. A reduction in early diastolic mitral annular velocity (E’) and an increase in E/E’ were observed in patients with mild to moderate asymptomatic AS, and related to the severity of AS. These measures have been reported to correlate with left ventricular filling pressures and may be a useful predictor of developing symptoms.46 These findings demonstrate that the damage to the left ventricular myocardium is an on-going process, which starts long before the need for AVR according to the current guidelines.
Impaired left ventricular GLS (LVGLS), as a potential surrogate of myocardial fibrosis, has been shown to be an independent predictor of mortality and adverse outcomes in both symptomatic and asymptomatic severe AS patients, suggesting that LVGLS is a better value for risk stratification and management of AS, than LVEF.47,48
It has been shown that clinical outcomes of patients with moderate AS are poor, highlighting the importance of detecting even mild degrees of left ventricular dysfunction before the AS becomes severe. Zhu et al. retrospectively analysed data from 287 patients with moderate AS (aortic valve peak velocity [AVVmax] 3.2 ± 0.5 m/s, MG 24.5 ± 7.4, AVA 1.26 ± 0.14 cm2 and LVEF 62 ± 6%). They found that mortality was significantly higher in patients with GLS ≥15.2% compared with GLS ≤15.2%. LVGLS was independently associated with survival even after adjusting for age, sex, coronary artery disease, LVEF, MG and symptomatic status.49 Increased afterload could possibly be already overwhelming even if AS is only moderate among patients with impaired GLS. Patients with impaired GLS had larger left ventricular mass index with concentric left ventricular hypertrophy and lower E velocities compared to those with preserved GLS.
Moderate AS based on current echocardiographic data may be associated with reduced survival, which in part may be related to comorbid clinical factors and co-existent cardiac abnormalities. Hence, the concept of staging AS and its relationship to clinical outcomes (rather than merely classifying the severity of the valve lesion based on echo criteria) may aid in the risk stratification of patients with AS. Genereux et al. characterised patients with symptomatic severe AS undergoing AVR into five stages: stage 0, no left ventricular damage; stage 1, left ventricular damage; stage 2, left atrial enlargement and mitral valve damage; stage 3, pulmonary hypertension and tricuspid damage; stage 4, right ventricular dysfunction has been proposed to aid the decision to potentially intervene in a more timely fashion. A main focus of these staging algorithms has been the changes that occur in the myocardium in AS and the pattern of left ventricular remodelling.50 Incorporation of LVGLS into the staging classification improves the prognostic value by identifying patients with more advanced cardiac damage.51
Including GLS (as a surrogate of myocardial fibrosis) in the echocardiographic evaluation of patients with AS, irrespective of its grade, may aid in the risk stratification of these patients. Early pressure unloading of the left ventricular can result in a more significant regression of diffuse fibrosis. The limitations of the current evidence are its retrospective nature and single centre study design. There is a need for a randomised clinical trial to determine whether patients with moderate AS and impaired GLS benefit from earlier intervention.
Exercise Echocardiography
Exercise stress echocardiography allows not just the assessment of functional capacity and haemodynamic changes, but also the measurement of trans-valvular gradient under higher flow conditions, measurement of left ventricular contractile function and estimation of pulmonary artery systolic pressures. Little is known about how the ventricle and vasculature respond to less than severe AS and whether this could lead to symptoms in susceptible individuals. More information may be revealed about the ventricle’s adaptation/maladaptation when placed under even mild stress compared to being at rest.
Moderate AS is considered a pre-symptomatic stage although there is a strong suggestion that a proportion of patients are already symptomatic; up to 60% exhibited breathlessness on exertion in one study,52 and that these symptoms on exertion are more closely linked to outcome than resting echo based severity parameters. Changes in exercise performance in patients with moderate AS are not well understood although there is evidence to support a reduction in exercise time of around 5% over 2.5-year follow-up.53 A small study of 38 apparently asymptomatic patients with moderate-to-severe AS revealed symptoms were associated with a lower peak VO2 on cardiopulmonary exercise testing, and lower peak stroke index during exercise. The limitation of the current evidence is that the cohorts either contain patients with both moderate and severe AS or have incomplete exercise data. Furthermore, the exercise data are confined to relatively crude treadmill parameters such as exercise time, but they do not establish the pathogenesis of the exercise limitation.
An abnormal left ventricular response to exercise (manifest by a lack of increment or decrease in LVEF on exercise) is associated with an increased likelihood of developing symptoms on exercise and lower survival free of cardiac events than those with an appropriate increase in LVEF on exercise. However, left ventricular longitudinal strain is a more powerful parameter than LVEF to predict the occurrence of symptoms, exercise tolerance and cardiac events. Huded et al., in a study of 504 asymptomatic patients with severe AS, showed that age and sex predicted metabolic equivalents (HR 1.16), LVGLS and valvuloarterial impedance, offered incremental prognostic value.54 However, at present, no clinically applicable cut offs for contractile reserve using strain in AS have been validated.
Exercise stress echocardiography using tissue Doppler imaging also provides prognostic information, in mild to moderate AS. A significant increase in the E/E’ ratio during exercise predicted adverse cardiac events, beyond exercise echocardiography or exercise testing alone.55 This highlights that the increased afterload alters the ventricular filling dynamics as well as the relaxing properties of the left ventricle in AS patients. The data demonstrates that objective assessment of the myocardium and valve behaviour under stress conditions, may aid in the decision making regarding aortic valve intervention, before irreversible myocardial changes occur.
Cardiopulmonary exercise testing provides an additional objective measure of exercise tolerance and has been shown to be feasible and reproducible in AS and able to identify a greater proportion of individuals with exercise intolerance than conventional parameters that are recommended in the current guidelines (symptoms or fall in blood pressure).56–58 Previous studies have shown that a significant proportion of patients with apparently asymptomatic moderate-to-severe AS exhibit a peak VO2 <84% predicted.58,59 A reduced peak VO2 was associated with a lower event-free survival compared to a normal peak VO2. No exercise stress echocardiography parameters however, were associated with peak VO2, nor predicted event free survival.59 Whether patients with purely moderate AS exhibit exercise limitation defined by a reduced VO2 peak is yet to be established.
Cardiac CT and PET
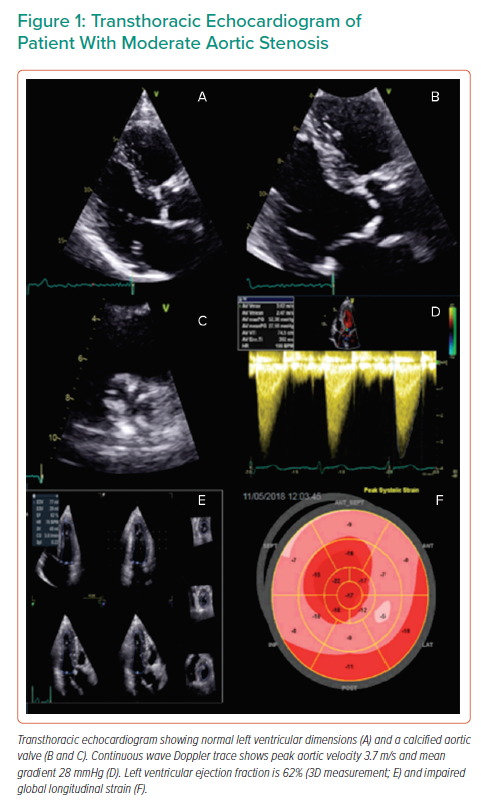
Quantification of aortic valve calcium (AVC) has been shown in multicentre studies to be reproducible and offers prognostic value above and beyond echocardiographic indices of AS severity (Figure 2).60,61 AVC load can be correlated with haemodynamic indicators of the severity of AS, as demonstrated by Boulif et al. in a study of 266 patients with moderate-severe AS (defined by an effective orifice area of <1.5 cm2), thereby potentially providing a valuable surrogate marker of AS severity.62 AVC load may predict outcome, including in asymptomatic patients, independently of other markers that may be detected on echocardiography, such as AVA and EF. The possible distortion of the former by reduction of the latter, whereby reduced LVEF may impact on the AV gradient and thereby result in functionally low AVA, renders the use of independent markers of AS severity all the more crucial in order to plan interventions of the most appropriate means and timing.63–65 The inclusion of CT-AVC in guidelines for the assessment of AS severity provides the optimal framework with which to apply this marker to clinical practice.2
A further interesting application of CT in AS is the use of hybrid PET-CT imaging to track both valvular inflammation and calcification with 18F-flurodeoxyglucose and 18F-fluoride-PET-CT. Earlier studies have suggested that 18F-NaF activity predicts the rate of future disease progression as measured by CT-AVC and echocardiography and prospective studies are also underway to assess whether it can improve prediction of risk and response to therapy.65,66
Cardiac MRI
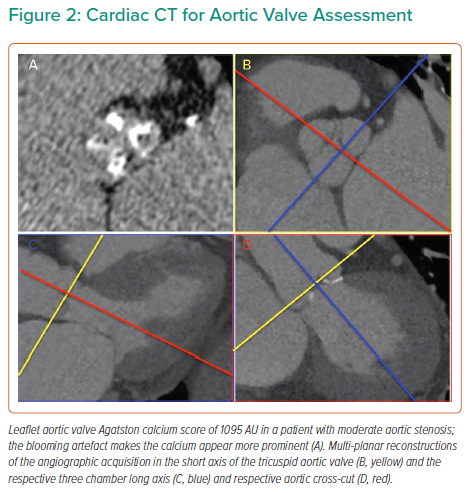
The importance of the transition from physiological adaptation to pathophysiological maladaptation in the myocardial remodelling response in AS was highlighted over 40 years ago in pivotal work by studying myocyte hypertrophy and fibrosis on myocardial histology. Work by Schwarz and others demonstrated proportional myocyte hypertrophy and diffuse interstitial fibrosis, as well as partly disease specific patterns of fibrosis, described as compact or ‘focal’, perimyseal, perivascular, plexiform or patchy.67 In AS, interfibre and perivascular fibrosis increase disproportionally.67 Later work also demonstrated gradients of fibrosis and capillary rarefaction from the subendocardium than in the subepicardium.68,69 Fast forward, we can now identify many of these changes non-invasively by cardiac MRI (CMR) using not only the superior accuracy of CMR to quantify left and right ventricular size, hypertrophy and function (Figure 3) but also quantifying both focal scar (with late gadolinium enhancement [LGE] imaging) and diffuse fibrosis (with T1 mapping that allows the quantification of the extracellular volume fraction (ECV%). Both techniques have been validated against myocardial fibrosis on histology highlighting the previously described complex pattern of subendocardial microscars and mid-myocardial diffuse fibrosis.70–72 Importantly, focal scar can already be detected in moderate AS. Everett et al. showed both the amount of LGE and elevation of ECV% increases in moderate AS prior to left ventricular impairment or symptom onset with new scar occurring both at the sites of existing LGE and, in a quarter of patients, at remote sites with the development of new areas of non-infarct LGE.73 Both LGE and ECV% are independent predictors of outcomes after AVR, but importantly LGE is irreversible, whereas diffuse fibrosis quantified by ECV% regresses after AVR.74–77
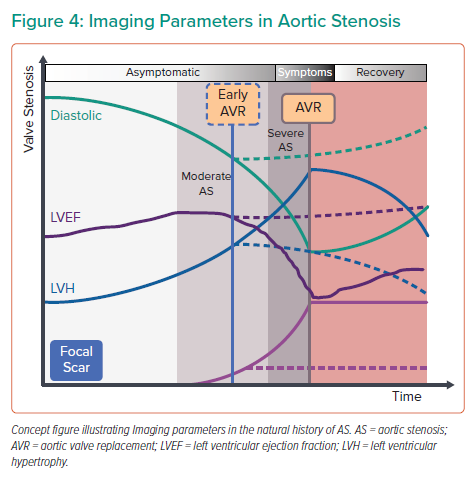
Whether myocardial scar can be used to guide early AVR in asymptomatic severe AS, is currently under investigation in the EVOLVED study, which is the first multicentre randomised controlled trial to compare early AVR to routine care in asymptomatic severe AS guided by the presence of focal scar on LGE imaging.78 If this study provides a role of CMR in guiding timing of intervention, then the next conceivably are of CMR-guided early intervention will be moderate AS with evidence of myocardial decompensation (Figure 4). The increased use of CMR has also highlighted dual pathology AS and transthyretin-type cardiac amyloidosis, which occurs in one in eight elderly patients referred for TAVR.78,79 Although TAVR is beneficial in AS-amyloidosis, diagnosis is still important because new amyloid-directed pharmacotherapies are now available and should be considered. Beyond fibrosis and infiltration, CMR can also detect myocardial perfusion reserve and oedema, with studies on-going to establish the importance of microvascular perfusion and low grade of inflammation in the pathophysiological remodelling of AS.
Just a Question of Classification?
The ultimate question is whether we need to re-classify AS bearing in mind the importance of the myocardial response and its impact on outcome, and the improved outcomes with better surgical and trans-catheter technology (that lower the upfront risk and change the balance of risk-versus-benefit). While the label ‘severe’ signals the need for intervention, we should aim to describe the valvular insult to the myocardium and upstream cardiac structures as significant or non-significant and then assess the resultant response.
How classification can have a counterproductive effect can be seen by the strict interpretation of the European Society of Cardiology (ESC) 2017 guidelines that reclassified low-gradient severe AS to moderate AS unless presenting with low flow status (stroke volume index ≤35 ml/m2) and high calcium scores (emphasising on mean pressure gradient and an integrated approach). Chan et al. demonstrated that strict adherence to 2017 ESC guidelines for valvular heart disease reclassified 45% of their severe asymptomatic severe AS cohort to moderate (PRIMID-AS) and that both the severe and reclassified groups had a higher risk compared with moderate AS with the reclassified group demonstrating an intermediate risk.80 This work from the PRIMID-AS investigators demonstrates that moderate AS clearly is in grey area where exercise testing, multimodality imaging, and possibly lower AS severity cut-offs may personalise the treatment of AS and may improve risk stratification.
Conclusion
Moderate AS carries a high morbidity and mortality and there is evidence to suggest that these patients may benefit from earlier intervention. The current evidence highlights the importance of careful surveillance of these patients. However, small patient numbers and the retrospective study nature limit much of the evidence. Echocardiography is pivotal in guiding management, although alternative imaging modalities such as CT, CMR and PET are increasingly being used to assess disease activity and progression. A new era of randomised clinical trials is required to evaluate the hypothesis that imaging-driven care in earlier stage aortic valve disease with upstream intervention would result in better long-term clinical outcomes.
Clinical Perspective
- Aortic stenosis is the most common left-sided valve lesion requiring intervention and carries a substantial morbidity and mortality burden.
- Clear guidelines exist for the management of patients with symptomatic severe aortic stenosis.
- However, patients with moderate aortic stenosis may also experience symptoms, to which no other cause can be attributed to other than the valve.
- It is well recognised that aortic stenosis is not only a disease of the valve, but also of the myocardium, and that a significant proportion of patients with moderate aortic stenosis will also have left ventricular dysfunction.