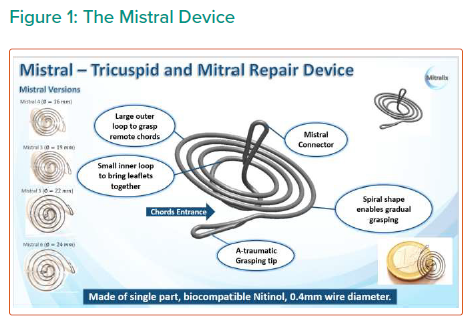
Objective: Present Echo CoreLab (Prof Topilsky) analysis short term results of all tricuspid first-in-human study Mistral device (Mitralix) performed up to October 2020.
Methods: Trans-thoracic echocardiography (TTE) files were sent to Echo CoreLab for analysis. Echo CoreLab was blinded to sites and results of all exams. Measurements include tricuspid regurgitation (TR) parameters and right ventricular function parameters at 1 month and 6 months follow-up.
Results: 17 Mistral procedures were performed in four different centres (Germany and Israel). Results of 46 TTE exams are presented in this poster.
Conclusion: The Mistral device (single or multiple devices) was seen in TTE and TR and right ventricular (RV) function parameters can be measured. It was concluded that TR and RV function parameters improved at 1 month and 6 months.
Introduction
Methods and Materials
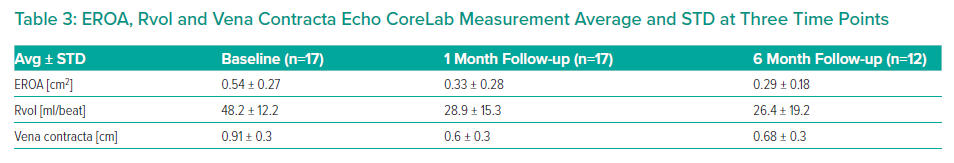
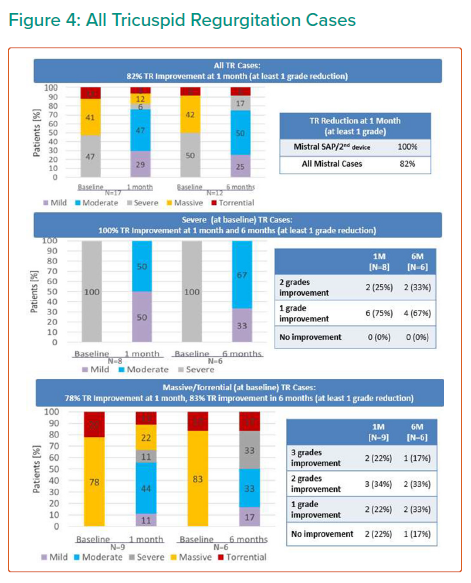
Tricuspid regurgitation parameters are graded using a five-class grading scheme (mild, moderate, severe, massive, and torrential) according to the standard American Society of Echocardiography grading scheme.3 Grading of TR parameters severity (mild, moderate, severe, massive and torrential) followed recommendations given in current guidelines with consideration of qualitative parameters, semi-quantitative parameters, and quantitative parameters. The expanded grading scheme is used in order to take into account the massive and torrential grades. Cut-off values are 21 mm, 80 mm² and 75 ml for vena contracta width, EROA and Rvol by PISA, respectively, with values equal or higher indicating torrential TR.
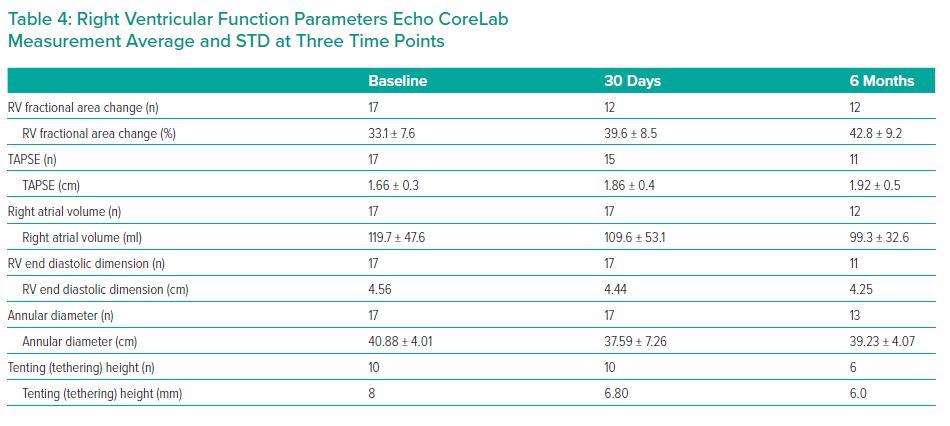
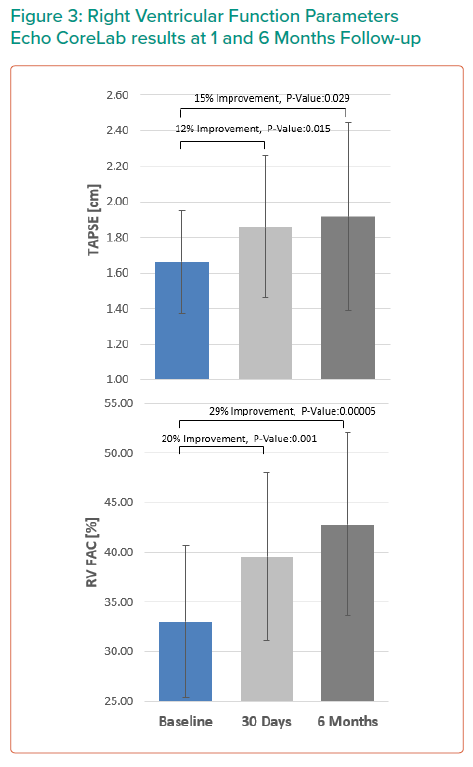
RV function was assessed using several parameters, including RV fractional area change (RV FAC), Tricuspid Annular Pane Systolic Excursion (TAPSE), right atrial volume, RV end diastolic dimension, annular diameter and tenting height.4
Results
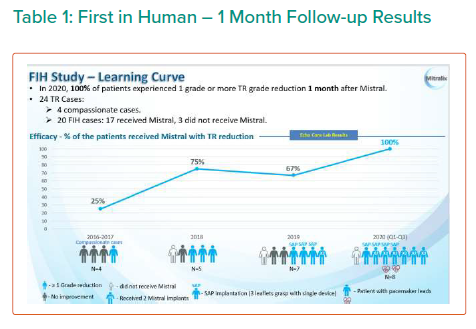
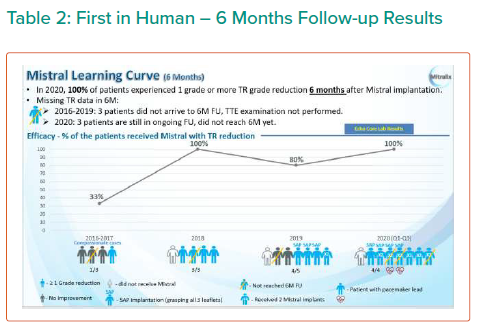
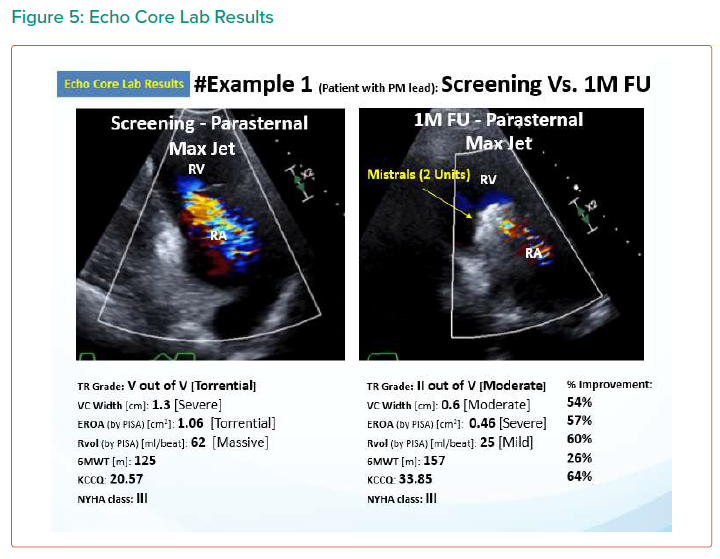
The results are summarised in Tables 1, 2 3 and 4 and Figures 3, 4 and 5 on the following pages.
Discussion and Conclusion
Echo CoreLab analyses include 46 exams, for 17 patients, from 4 different sites at 3 time points (baseline, 1 month and 6 months). The Mistral device (single/multiple) was clearly seen in TTE in all follow ups. TR results shows improvement in 1 months follow up and durable up to 6 months follow up. The Mistral device provided, beyond substantial TR reduction, a clinically important improvement in RV function and significant RV reverse remodelling. Further studies are required to show safety and efficacy with the innovative novel Mistral device.