Aortic valve stenosis is the most common valvular lesion in Australia, with a rising prevalence in line with the ageing population.1 The number of Australian and New Zealanders aged >65 years will increase by 25% between 2020 and 2027.2,3 The most robust recent modelling has estimated a 4.4% annual incidence of severe aortic stenosis in this population.4 Aortic stenosis is characterised by progressive thickening, fibrosis and calcification of the aortic valve leaflets leading to restriction and valve obstruction.5 Clinical manifestations of severe aortic stenosis include angina, dyspnoea, syncope and heart failure.1 If left untreated, symptomatic severe aortic stenosis has an extremely poor prognosis, with a 30–50% mortality at 12 months.1 Traditionally, treatment has entailed surgical aortic valve replacement (SAVR), but many patients deemed excessively high risk for an open surgical procedure are left untreated.
Transcatheter aortic valve implantation (TAVI) has generated a worldwide paradigm shift in the treatment of severe symptomatic aortic stenosis. TAVI initially emerged as a novel alternative treatment modality to SAVR in patients with multiple comorbidities at high risk of surgical complications, originally reserved for carefully selected inoperable patients with very high surgical risk.6 Subsequently, data have emerged on the safety and efficacy of TAVI in sequentially lower risk cohorts.6–9 Despite the PARTNER trials continuously demonstrating the efficacy of TAVI versus SAVR in consecutively lower risk cohorts, the current publicly funded universal healthcare insurance scheme in Australia – known as Medicare – requires a patient to have severe symptomatic aortic stenosis and an unacceptably high surgical risk, as assessed by a TAVI case conference including a cardiothoracic surgeon, interventional cardiologist and non-procedural physician.6–10 However, there are current applications by Medtronic, Edwards Lifesciences and Abbott Vascular to the Australian Medical Services Advisory Committee to approve public funding for their respective valves in intermediate- and low-risk patients.
The Funding Model, Accreditation and Heart Teams
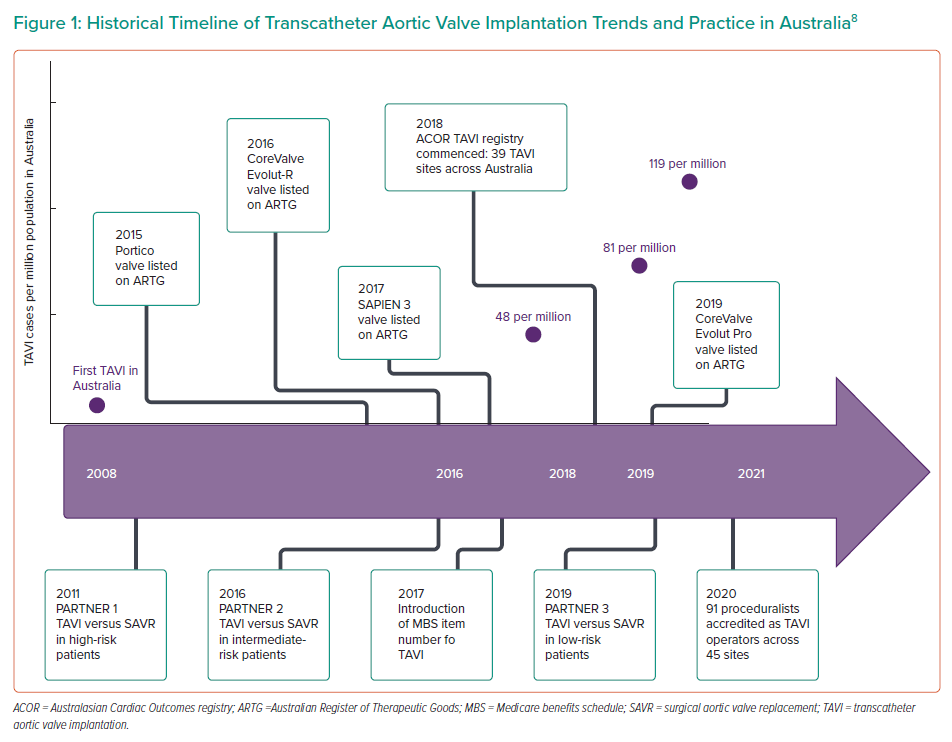
TAVI was first performed in Australia in 2008 as part of early clinical trials, but growth of this therapy has been slow compared with other developed nations because of high prosthesis costs and delays in Medicare funding for the procedure.11 Until recently, funding for TAVI has required individual hospital or health service arrangements – often directly with industry or via clinical trials – to be able to offer the service. The introduction of a Medicare benefits schedule item number in November 2017 has made TAVI rebateable and thus more accessible to patients and providers across the Australian healthcare system. Specifically, the indication chosen was, and remains, individuals who have severe symptomatic aortic stenosis and are of prohibitive or high surgical risk and ultimately deemed suitable for TAVI by a heart team.10
This has resulted in rapid growth of the number of TAVI sites, operators and procedures being undertaken in Australia, with the number of implanting sites growing from seven in 2008 to 45 in 2020, with 91 proceduralists now accredited as TAVI operators.11,12 Approximately 50 TAVIs were performed in Australia in 2008, with the number having grown to more than 1,000 by 2018 with a yearly growth of 30–40%.11 Between April 2018 and May 2020, 4,098 TAVI procedures were performed in Australia.12
Despite the recent acceleration in TAVI numbers, the uptake of TAVI in Australia has been relatively slow compared to that of the northern hemisphere, and SAVR continues to be the dominant form of aortic valve intervention, with only 6–10% of all aortic valve procedures being performed using a transcatheter approach between 2013 and 2015.13 TAVI continues to be restricted by limited public funding that caps the number of procedures able to be offered at each centre per year. The penetration of TAVI in Australia has increased from 48 cases per million in 2016, to 81 cases per million in 2018, and 119 cases per million in 2019.12 In comparison, current TAVI procedural rates for symptomatic severe AS have reached over 200 per million in many European countries, while in the US the number of TAVI procedures exceeded the number of all SAVR procedures in 2019, according to the latest transcatheter valve therapy registry data.14
In view of this increase, the Cardiac Society of Australia and New Zealand and the Australia and New Zealand Society of Cardiac and Thoracic Surgeons have provided guidance on training requirements for centres contemplating TAVI programmes. To ensure ongoing high standards of care, a national TAVI accreditation committee was set up, which requires hospitals and implanting cardiologists to go through a rigorous accreditation process to perform TAVI and monitors procedural volumes and audits patient outcomes. Institutions and individuals are expected to achieve outcomes that are consistently within two standard deviations from the average outcomes of peer institutions in the TAVI registry and are required to have a 90% submission rate of complete data, including 1-year follow-up, to the National TAVI registry.12 Current accreditation of TAVI operators in Australia requires performance of 30 TAVIs (as primary or secondary operator) and 10 initial proctored cases.15
Recent data demonstrate that patient outcomes relate to TAVI volume in the early site experience, although the learning curve effect dissipates after 200 cases.16 This supports the importance of heart teams and proceduralists having appropriate levels of experience. The current emphasis on the indication being patients at prohibitive or high risk for surgical AVR and the requirement for a heart team discussion are central tenets of the Australian TAVI experience. The mandated requirement for heart team discussion is now the cornerstone of TAVI clinical practice in Australia and is entirely consistent with recommendations in Europe, North America and the UK.14 It is anticipated that the role of the heart team will become particularly important with expansion of indications to lower risk groups. Heart team discussion is thought to minimise bias in patient selection, prevent indication creep and ultimately ensure optimal patient outcomes.1 The goal is for Australian patients to have access to heart team assessment in a safe and timely manner and not be denied access to aortic valve intervention on the basis of geographical or funding restrictions.
Valve Types and Procedural Techniques
Over the last 10 years in Australia, rapid progression has occurred in the dynamic field of TAVI, resulting in changes in patient cohorts and procedural techniques, as well as updated iterations of the transcatheter heart valves themselves. Specifically, the first-generation Edwards Lifesciences and Medtronic transcatheter heart valves that were implanted in 2008 under special access schemes have been replaced by the latest generation SAPIEN 3 Ultra and Evolut Pro and joined on the Australian market by the availability of Portico (St. Jude Medical) and Lotus (Boston Scientific). There has been a reduction in delivery sheath size from 21 Fr to 14 Fr, the provision of sealing cuffs, improvements in deliverability, the ability to reposition and retrieve, a move to a minimalist approach under local anaesthesia and a less aggressive approach to percutaneous coronary intervention pre-TAVI and revascularisation only for significant angina and critical, proximal disease.17,18
In addition, safety has been facilitated by developments in imaging and its analysis, particularly using CT scanning. This has led to more accurate determination of device sizing, which may improve device apposition and reduce the risk of annular rupture or coronary occlusion. Pre-procedure CT scanning can also accurately evaluate the luminal size, tortuosity and calcification of the iliofemoral arteries and reduce vascular and bleeding complication related to femoral access.19 Additional factors that will need to be considered in future younger patient cohorts include the extent of concomitant coronary disease and potential need for future revascularisation, the presence of an associated aortopathy in patients with congenitally bicuspid aortic valves, the long-term consequences of pacemaker implantation and options for re-do or surgical AVR in the event of prosthetic valve degeneration.12 Delivery sheaths, vascular access techniques, implant depth and patient selection criteria have all undergone massive shifts over the last 10 years in Australia leading to improved safety and outcomes of the procedure.
TAVI is now performed with conscious sedation, without the need for intra-procedural transoesophageal ultrasound, reducing the number of staff needed and the requirement for post-procedure intensive care, making TAVI a more cost effective, time efficient and less labour-intensive procedure for the Australian healthcare system (Figure 1).
Local Outcomes
A multicentre prospective cohort study of 540 patients across eight Australian hospitals and two New Zealand hospitals between 2008 and 2013 described the early Australian experience with the Medtronic CoreValve system for patients with symptomatic severe aortic stenosis.20 This study included initial use of the CoreValve system for all investigators. They found a mean patient age of 84 years and mean Society of Thoracic Surgeons (STS) score of 5.7%. At 2 years, all-cause mortality was 21.2%, cardiovascular mortality 15.2%, and stroke 10.1%. The rate of permanent pacemaker implantation was 28.4% at 30 days and 29.4% at 2 years. All-cause mortality was found to be similar to that in the UK TAVI registry.21 However, major vascular complications, bleeding and permanent pacemaker (PPM) rates were higher than other CoreValve studies. This was possibly explained by this study cohort including the learning curve of all investigators, and the addition of the AccuTrak Delivery System and more consistent use of CT for valve sizing to later enrolments towards the end of the study period.
In 2018, the Australasian cardiac outcomes registry (ACOR) TAVI registry was commenced encompassing 39 TAVI sites across Australia, with the goal of quality control and monitoring of procedural and clinical outcomes of patients undergoing aortic valve replacement via a transcatheter approach. Currently 43 sites across Australia are included in the ACOR registry (25 private hospitals and 18 public hospitals). Early registry data were published in 2019, demonstrating that 865 procedures had been undertaken since commencement of the registry, with the majority (81%) being performed in the private sector.22 The mean patient age was 83 years, mean STS score 5.87% and average length of stay 4 days. Mortality, adverse event rates and patient reported outcome measures were comparable to other international registries.22
A recently published multi-centre Australian cohort of 601 patients who underwent TAVI for severe symptomatic aortic stenosis between 2008 and 2018 found a trend to lower risk patients undergoing TAVI.23 They found mean patient age was 84, with 47% deemed low risk (STS <4%) and 40% intermediate risk (STS 4–7.9%) with only 12% deemed high risk according to STS score. Again, this cohort reported adverse events and outcome measures comparable to other international registries and – importantly – showed no difference in pacemaker insertion rates between groups, which represents a significant on-going hurdle for TAVI in low-risk populations.
Most recently, the SOLACE-AU trial was published in 2020, which was a multicentre, prospective clinical trial on 199 consecutively enrolled intermediate risk Australian patients who underwent TAVI with the SAPIEN XT (Edwards Lifesciences) transcatheter heart valve.24 Mean patient age was 85.5 years with a mean STS score of 5.9%, and results compared favourably with the outcomes of the PARTNER IIA trial.8 At 2 years, all-cause mortality and cardiovascular mortality were 16.8% and 8.8%, respectively. Stroke, major vascular complications and the new PPM rate at 30 days were 3.5%, 6% and 8%, respectively.24
Recent excellent outcomes have also been achieved in the private system in Australia, illustrated by a recently published single centre cohort of 300 consecutive patients undergoing TAVI between 2015 and 2018.25 Median age was 85 years with a mean STS score of 4.0%. Peri procedural complication rates were low with a major vascular complication rate of 3.0%, new PPM rate of 9% and no life-threatening or disabling bleeding. At 1 year, mortality was 4.2%, stroke 2.1%, MI 0.3% and PPM rate 11.4%.25 As discussed above, the current funding model in Australia presents unique challenges to service delivery, but excellent outcomes have been demonstrated via both public and private hospitals in the country.
Access to Care and Service Delivery
The land mass of Australia is 32 times the size of the UK, yet the population of the UK is almost three times that of Australia.2 This results in a geographically dispersed population, which provides difficulties in providing a TAVI service to regional and rural Australians. Australians living in regional and remote areas have inferior health outcomes compared with their urban counterparts.26 Australian regional and rural health services have limited access to invasive therapies, such as for acute coronary syndromes and patients require lengthy transfers to tertiary referral centres, which might delay definitive treatment.27,28 Despite this, equitable outcomes have been achieved in rural patients undergoing TAVI in Australia.
A single-centre study of 142 patients consisting of 54% from regional Australia and 13% from outer regional Australia found no differences in procedural success and 30-day or 12-month mortality rates between regional and urban patients.29 Importantly, these were regional patients who had to travel to a tertiary referral hospital to undergo their procedure.
More recently, a TAVI programme was developed at a geographically isolated tertiary hospital in Townsville in regional Australia. A total of 19 patients underwent TAVI over a 12-month period with zero major vascular complications, stroke, PPM insertion or mortality, and a 10% incidence of moderate paravalvular leak at 30 days.30 This small, single-centre study outlined the safe and effective implementation of a TAVI programme in a regional tertiary hospital in Australia.30
These outcomes may support other regional centres in the introduction of a TAVI service. However this needs to be balanced with recommendations that TAVI should take place in major tertiary centres with on-site cardiac surgery, interventional radiology and intensive treatment units to appropriately manage complications as well as extensive operator and site experience, which has been shown to be an important predictor of patient outcomes.14,16
Conclusion
TAVI outcomes have significantly improved over time in Australia with operator experience, improved patient selection and new device technology including second-generation valves and delivery systems. The focus within Australian heart teams and health systems has shifted from how to technically perform TAVI to how we select individuals most likely to benefit from the intervention. With applications pending to perform TAVI in lower risk patient cohorts and restricted public funding for the procedure, deciding who is appropriate for intervention will continue to be a challenge for structural heart teams now and into the future. Further intra-procedural and device-related improvements should continue to drive transcatheter technology into the future and ultimately see TAVI become the gold standard for most patients with severe aortic stenosis in Australia and abroad.










