Atherosclerosis is the basis of adverse cardiovascular events. It has been shown that endothelial damage and inflammation play critical roles in the development and progression of atherosclerosis. Endothelial dysfunction refers to an impairment of the ability of the endothelium to properly maintain vascular homeostasis. Although the term is often used in reference to a loss of bioavailable nitric oxide, endothelial dysfunction also reflects increased production of vasoconstrictors and disturbed regulation of inflammation, such as adhesion molecules, chemokines and leucocytes to enhance low-density lipoprotein oxidation, platelet activation, and vascular smooth muscle cell proliferation and migration thrombosis, and cell growth in the vascular wall, which causes macrovascular and microvascular dysfunction.1
Numerous studies have linked endothelial dysfunction and resultant atherosclerosis with insulin resistant states, such as obesity and diabetes.1 Diabetes is associated with an increased risk of cardiovascular disease. Substantial clinical and experimental evidence suggest that both diabetes and insulin resistance cause a combination of endothelial dysfunctions, which may diminish the anti-atherogenic role of the vascular endothelium. Statins have been attributed to a range of beneficial actions, but their capacity to inhibit endothelial dysfunction and their anti-inflammatory effect probably generated the most interest.1 At the same time, LDL is also involved in this process due to its vasoconstrictive, mitogenic, pro-inflammatory and thrombogenic effects. Statins are medications that alter cholesterol metabolism by inhibiting the rate-limiting enzymes involved in cholesterol biosynthesis, thereby reducing the level of circulating cholesterol. Therefore, statins are used for primary and secondary cardiovascular event prevention.2–5
According to the 4S study on the role of statins in the secondary prevention of adverse cardiovascular events, statins can significantly decrease the plasma levels of LDL and total cholesterol, while increasing the HDL level.6 Therefore, statins can decelerate atherosclerosis progression.
In contrast, chronic coronary syndrome (CCS) is caused by an increased myocardial oxygen demand in the presence of a stable atherosclerotic plaque.7 Therefore, coronary angioplasty with intravascular stent placement is the most common method for improving myocardial perfusion in patients with significant coronary artery disease (CAD) and CCS, even in patients with stable conditions.2–5
Clinical trials investigating statins have shown the role of this group of medications in preventing MI during therapeutic interventions, such as percutaneous coronary intervention (PCI). Major adverse cardiovascular events (MACEs) are important causes of mortality and morbidity in cardiovascular patients with a history of PCI, because patients with a cardiovascular history are at high risk of MACE development.4,5 Therefore, recent studies have investigated the sufficient dose of statins for cardiovascular event reduction in the short- and long term. However, due to insufficient evidence, recommendations for pre- and post-PCI statin doses cannot be generalised to Asian populations.7
Currently, there is no clear evidence to show the effect of statin dose on myocardial perfusion and complications after PCI, and the optimum dose and duration of statin use remain a major dilemma. Hence, the present study intended to investigate the effective statin dose for MACE prevention in patients with CCS following PCI.
Methods
The present study was a randomised, double-blinded clinical trial. The study population included patients with CCS who had undergone recent PCI and presented to the Shiraz Central, Al-Zahra Heart, Kowsar, Dena, and Martyr Dr Faghihi Hospitals of Shiraz, Iran. The patients were referred to the Professor Kojuri Cardiology Clinic for their first post-intervention follow-up visit 1 month later during 2020 and 2021.
Patients who were willing to participate provided informed consent. Then, they were divided into two study groups using permuted block randomisation with size four blocks. Different permutations were assigned to the number 1–6 as follows: AABB, ABAB, ABBA, BBAA, BABA and BAAB. Then using a random digits table, we selected a number from 1 to 6, and then the patients were randomly divided to treatment group A or B.
The participants in both groups were administered 40 mg of daily rosuvastatin post-PCI for 1 month. After 1 month, a median of 28 days (range 24–37 days), after the first clinic visit, the rosuvastatin dose was changed to 5 mg daily for 1 year in the first group, while the second group received the previous 40 mg daily dose for the same duration.
The demographics and other data of the patients, including age, sex and BMI, were recorded. Then, the participants were evaluated in terms of MACEs. A MACE was defined as any hospitalisation due to acute coronary syndrome (ACS), stroke, MI, unplanned revascularisation or cardiac death.8
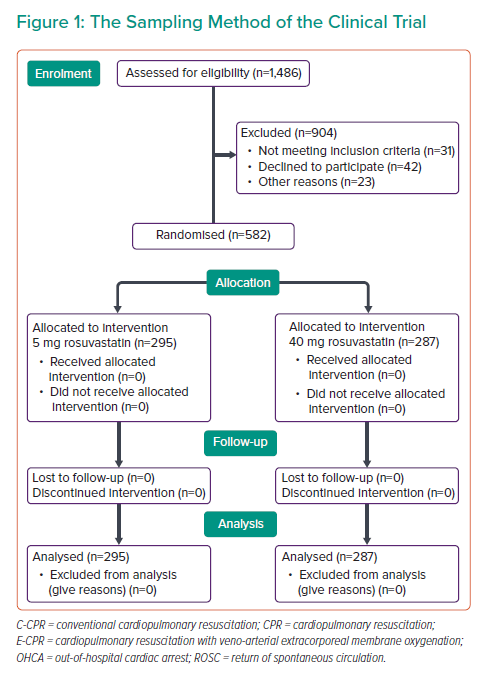
Considering the study design, objective and experiences from previous studies, 1,486 participants entered the study.9 According to the inclusion criteria, all patients who had undergone PCI due to CCS entered the study. However, 808 of these participants were excluded due to fulfilling the exclusion criteria. The exclusion criteria were: patients with ACS, primary LDL >190 mg/dl, patients who developed statin-induced myopathy or rhabdomyolysis, persistent increase in hepatic enzyme levels or hepatic failure, pregnancy and breastfeeding, statin hypersensitivity, a history of multiple atherosclerotic events, and a history of receiving high-intensity statins before the study enrolment.
The study protocol was approved by the University ethics committee, and all Declaration of Helsinki ethical standards were adhered to. All participants were introduced to the study design and gave written consent.
Finally, a total of 582 patients were included in the study (Figure 1). The descriptive statistical indices were used for data description, including mean and SD for quantitative variables, and frequency and percentage (%) for qualitative variables. The paired t-test was used for comparisons between the mean values of variables in the groups. The SPSS software version 22 (IBM Corporation) was used for data analysis.
Results
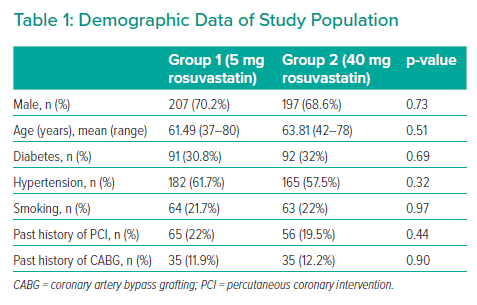
The study included 582 patients divided into groups: group 1 (n=295), which received 5 mg rosuvastatin daily, and group 2 (n=287), which received 40 mg rosuvastatin daily. The mean age of the participants was 63.09 ± 10.08 years and 62.19 ± 10.15 years in groups 1 and 2, respectively. In the 40 mg rosuvastatin group, eight patients (2.78%) developed a rise in fasting blood sugar at the level of impaired fasting glucose corrected by lifestyle modifications, nine patients (3.13%) developed an increase in alanine transaminase (ALT), and 15 patients (5.22%) presented with myalgia and myopathy. Those with rising ALT levels or significant myopathy were shifted to the 5 mg rosuvastatin group. However, these patients were considered members of the 40 mg statin group based on the intention-to-treat strategy. MACEs occurred in only three patients in the shifted group just before changing the rosuvastatin dose.
In contrast, in the 5 mg rosuvastatin group, five patients (1.69%) presented with rises in fasting blood sugar (at the level of impaired fasting glucose, corrected by lifestyle modifications), five (1.69%) developed an increase in ALT, and seven (2.37%) presented with myalgia and myopathy. Rosuvastatin was discontinued in those with significant ALT elevation or myopathy, and the final data were analysed with the intention-to-treat strategy. Nonetheless, MACEs did not occur in these patients (Table 1, Figure 1).
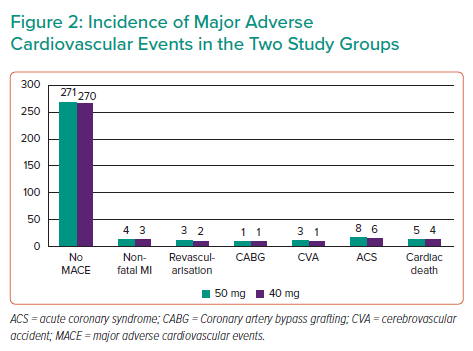
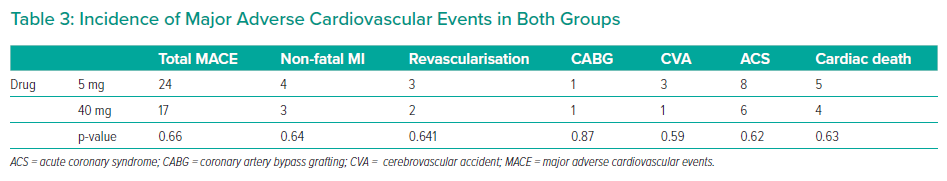
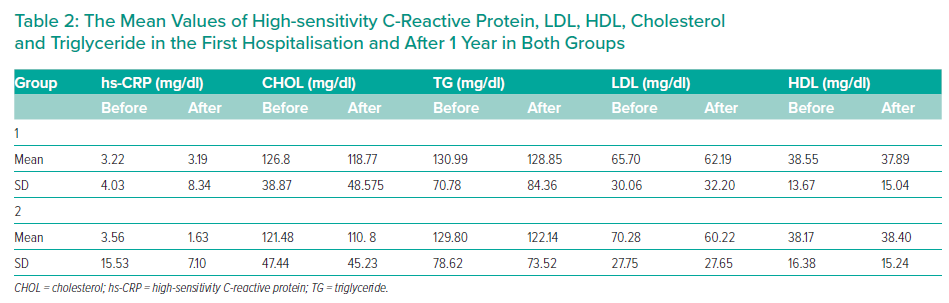
The groups were not significantly different in age (p=0.51), sex (p=0.73), hypertension (p=0.32), diabetes (p=0.69), smoking status (p=0.97), previous history of PCI (p=0.44), prior history of coronary artery bypass grafting (p=0.90) and SYNTAX II score. The mean SYNTAX score II was 22.4 and 21.7, in the 5 mg and 40 mg groups, respectively (p=0.86; Table 2). MACEs occurred in 24 patients in the 5 mg rosuvastatin group and in 17 patients in the 40 mg rosuvastatin group (Figure 2, Table 3). No significant intergroup difference was found in MACE occurrence during the 1-year follow-up (p=0.66). Furthermore, no significant intergroup difference was found in non-fatal MI (p=0.64), revascularisation (p=0.641), coronary artery bypass grafting (p=0.87), stroke (p=0.59), ACS (p=0.62) and cardiac death (p=0.63), separately. The basic criteria of patients with relapses are shown in Supplementary Table 4.
There were no differences in the p-values between the two groups’ lists of drugs regarding the drugs other than rosuvastatin, which is shown in Supplementary Table 5.
Over the intervention period of 1 year, cholesterol and LDL levels fell to a greater extent in the 40 mg rosuvastatin group, although the decrease was significant in both groups. In contrast, triglyceride reduction was only significant in the 40 mg rosuvastatin group (p=0.047). According to Table 2, high-sensitivity C-reactive protein (hs-CRP) levels decreased to an insignificant degree in both groups (p=0.713 in 5 mg group, p=0.637 in 40 mg group). Nonetheless, a greater decline was seen in hs-CRP in the 40 mg rosuvastatin group.
Discussion
According to a study by Jia et al. in China, the use of statins after acute MI could reduce the risk of mortality, recurrent MI and revascularisation.8 Moreover, a meta-analysis by Cannon et al., including four clinical trials, showed that high doses of statins as cholesterol-lowering agents could significantly improve the elasticity of large arteries and induced the regression of atherosclerosis.10 Also, another meta-analysis by Silva et al. including four clinical trials showed that high doses of statins could further prevent non-fatal cardiovascular events than the standard dose used for prevention. However, these high doses were associated with an increased risk of side-effects (OR 4.48; p<0.001).10 Given the meta-analyses investigating the side-effects of high-dose statins, the reduction of pre- and post-PCI MACE incidence by high-dose statins is still controversial. In the present study, the patients took 40 mg of daily rosuvastatin after PCI. After 1 month, the dose was reduced to 5 mg in 295 patients, while 287 patients continued the 40 mg daily dose for 1 year. Rosuvastatin use significantly improved the lipid profile in both groups; however, the MACE incidence and level of inflammatory phase proteins were not significantly different.
According to the studies by Neser Hossein et al., Ridker et al. and Kanadas et al. in Turkey, high and moderate doses of statins significantly reduced inflammatory phase protein levels, improved the lipid profile, and decreased MACE incidence and need for re-admission and revascularisation.11–13 Moreover, meta-analyses by Wang et al., Hao et al. and Baigent et al. showed that high-dose statins before PCI had significantly more benefits than a placebo in preventing post-PCI MACE incidence.14–16
A study by Rosendo, et al. investigated the effect of 20 mg daily simvastatin on individuals who were at high risk, moderate risk and low-risk for cardiovascular diseases. Similar to our study, they could not find a significant difference in hs-CRP levels between the group receiving simvastatin and those who did not receive simvastatin.17
The present study did not find a relationship between statin intake and MACE reduction in both groups. However, Tsai et al. investigated MACE development in patients with CAD. The study included 1,520 patients with CAD, of which 654 were affected by ACS and 855 had a history of PCI or stent placement. During a 32-month follow-up period, a total of 558 patients developed at least one MACE.18 Moreover, Jiang et al. evaluated the development of MACE in 90 patients. The participants received 40 or 10 mg of daily statin 1 week before PCI or were control cases with no intervention. The results showed that MACE development was significantly lower in the high-dose statin group than in the other groups.19
Farshidi et al. conducted a study similar to the present study and investigated the post-PCI MACE incidence during 1 year. The study included 192 patients, of which 93.8% underwent stent placement. Overall, 62% had only one stent, and the rate of successful angioplasty was 95.3%. No case of mortality during hospitalisation or emergency coronary artery bypass grafting was reported. However, eight patients developed MI.20 In the present study, 97% and 98% of the patients in the 5 mg and 40 mg groups underwent stent placement, respectively.
Kim et al. evaluated 171 patients with ST-elevation MI who were randomly divided into two groups. One group received 80 mg of daily atorvastatin before PCI, while the other group received 10 mg of daily atorvastatin. The MACE incidence was lower in the group receiving the lower dose of atorvastatin. However, compatible with our study, no significant intergroup difference was found.21
In a meta-analysis by Wang et al., 5,526 patients undergoing elective PCI were investigated. The study showed that high doses of statins before PCI could significantly reduce the development of MACEs.14 Another meta-analysis by Hao et al. evaluated the role of pre-PCI high-dose statin in reducing MACE development in CAD patients.15 The meta-analysis included five studies with 1,789 participants who were eligible for data analysis. High-dose statins in CAD patients before PCI were associated with a significant reduction in MACE development during 30 days post-intervention. Moreover, MACE development in the high-dose statin group (6.98%) was significantly lower than in the placebo group (14.77%). Therefore, high-dose statin before PCI was substantially more effective in preventing post-PCI MACEs.15
In another study, Montalescot et al. investigated adverse outcomes in 7,962 patients undergoing PCI.22 The study showed that the rate of adverse events during hospitalisation was low. The events included mortality (0.3%), MI (1%) and bleeding (3.4%). Angiography-related complications were observed in 8.7% of the patients.22
Limitations
Our study was a randomised, strictly controlled trial. However, larger population studies and investigations on ACS patients are needed to clarify the statin dose dilemma in depth.
Conclusion
We found no significant relationship between high (40 mg) or moderate (5 mg) doses of statins and MACE incidence reduction or decreased inflammatory factors, such as hs-CRP. However, the lipid profile significantly improved in both groups, and this improvement was better with the high-intensity statin. Considering the lack of difference in MACE reduction between high- and moderate-intensity statin therapy, it seems that the use of statins based on the LDL goal may be sufficient. Hence, we conclude that after the initial 30-day post-PCI high-intensity treatment period, continuing high-dose statins for 1 year in CCS patients is not mandatory.
Click here to view Supplementary Material.
Clinical Perspective
- There is no significant relationship between high (40 mg) or moderate (5 mg) doses of statins and MACE incidence reduction or decreased inflammatory factors, such as hs-CRP.
- Considering the lack of difference in MACE reduction between high- and moderate-intensity statin therapy, it seems that the use of statins based on the LDL goal may be sufficient.
- Continuing high-dose statins for 1 year in CCS patients may not be mandatory.
- The lipid level improved by both protocols, but was significantly lower with higher doses.