Dual antiplatelet therapy (DAPT) consisting of aspirin combined with a P2Y12 receptor inhibitor has been the standard of care for several decades in patients undergoing percutaneous coronary intervention (PCI) with implantation of coronary stents. According to current guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC), standard DAPT duration is defined as at least 6 or 12 months for stable coronary artery disease (CAD) and acute coronary syndrome (ACS), respectively.1 Standard DAPT as thus defined has been demonstrated to significantly reduce major adverse cardiovascular events (MACE) following PCI while increasing the risk for bleeding.2–5 Significant discourse in recent years has centred around variable DAPT duration for certain demographics based on risk stratification for both post-PCI MACE and bleeding, with numerous recent trials demonstrating noninferiority of shorter DAPT duration for low-risk patients.6–8
This article, complemented by an illustrative case, will review the current evidence for varying DAPT durations – with a focus on shortened DAPT duration – and the risk stratification strategies to appropriately identify candidates for short-term DAPT following PCI.
Illustrative Case
Mr Q was a 54-year-old man with a history of type 2 insulin-dependent diabetes, hypertension, hyperlipidaemia and recently diagnosed nonmetastatic left renal cell carcinoma who presented to the emergency room for new-onset decompensated heart failure. Mr Q was scheduled for nephrectomy with lymph node biopsy to be completed by urology within the following 2 months. During this time, he was also to undergo ischaemic cardiac workup for a monthslong history of exertional chest pain and shortness of breath. However, he had begun to develop worsening dyspnoea and lower-extremity swelling since his cancer diagnosis, leading to his current presentation. On admission, he was noted on echocardiogram to have new, severely decreased left ventricular ejection fraction (LVEF) of 13% and multiple segmental wall motion abnormalities. High-sensitivity troponin was mildly elevated to 23 ng/l. A coronary angiogram for further evaluation of ischaemic cardiomyopathy was indicated, raising questions of the potential duration of delay in his surgery because of the need for possible coronary revascularisation with stent implantation and DAPT.
History of Coronary Artery Stents and Anti-platelet Therapy
PCI, formally defined as coronary angioplasty with stent deployment, is the cornerstone of modern minimally invasive intervention for both CAD and ACS.9 Currently it remains one of the most widely performed procedures, with over 600,000 PCIs performed annually in the US alone.10
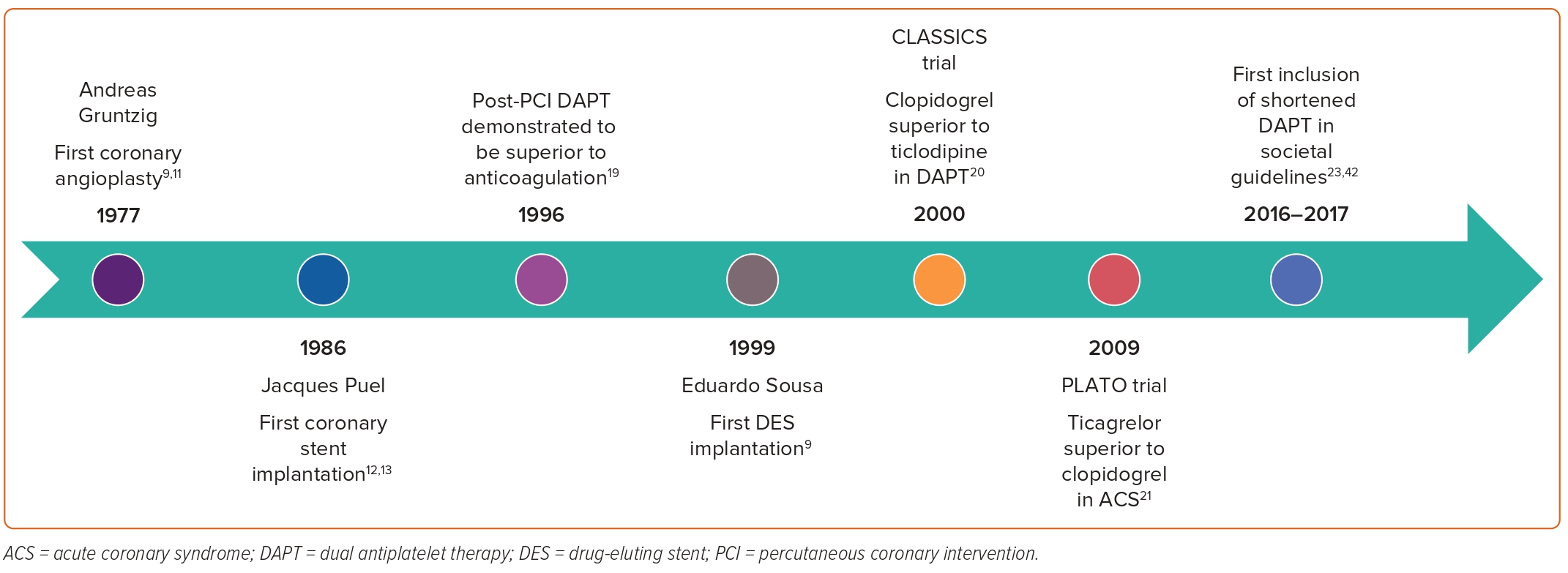
Interventional cardiology pioneer Andreas Gruntzig performed the first coronary balloon angioplasty on 14 September, 1977.9,11 Nine years later in 1986, French physician Jacques Puel implanted the world’s first coronary stent.12,13 In the decades since these two field-defining landmarks, the technique and technology behind PCI have seen rapid innovation (Figure 1). Following the publication of several large trials demonstrating the superiority of bare metal stent (BMS) deployment over angioplasty alone, the prevalence of stent implantation saw exponential increase throughout the mid to late 1990s.14–16 However, subsequent clinical follow-up of BMS PCI cases found a significant risk of in-stent restenosis (ISR) to an incidence as high as 30%, prompting research and development leading to the popularisation of drug-eluting stents (DES) at the turn of the century.16 Despite their success in reducing the incidence and complications of ISR, DESs carried their own risk of delayed endothelialisation, late stent thrombosis (ST), restenosis and neoatherosclerosis.17,18 These shortcomings were later mitigated by newer generations of DES and refinement of adjunctive antiplatelet therapy protocols.
Initial strategies to minimise the risk of ST centred around complex anticoagulation regimens using some combination of aspirin with heparin or warfarin, but these were associated with unacceptably high rates of major bleeding and vascular complications.16 Development of new anti-platelet therapeutics in the form of P2Y12 receptor inhibitors allowed for the breakthrough of DAPT, which was demonstrated to be superior to traditional anticoagulation agents as early as 1996.19 Combinations of DAPT initially included aspirin with ticlodipine, the latter of which was eventually supplanted by clopidogrel for improved tolerability and adverse effect profile.20 In 2009, the PLATO trial found that use of ticagrelor compared with clopidogrel was associated with improved cardiovascular outcomes in patients presenting with ACS; however this finding has been disputed by more recent data.21,22
The appropriate duration of DAPT therapy following PCI has seen similar movement over the last few decades, recently in a 2016 focused update to the 2011 ACC/AHA guidelines on DAPT duration that considered emerging evidence indicating the potential benefit of shortened DAPT duration for low-risk, non-ACS patients, followed by a more formal recognition of the same in the 2021 ACC/AHA/Society for Cardiovascular Angiography and Interventions (SCAI) coronary artery revascularisation guidelines.23,24 The appropriate length of DAPT for distinct patient demographics remains a topic of great importance and deliberation, and official guidelines across geographic regions continue to have minor discrepancies that reflect an evolving consensus on optimal therapy.
Recent Guidelines
ACC/AHA 2016 Focused Update on Duration of DAPT in Patients with Coronary Artery Disease
In March 2016, the ACC/AHA published a focused update to the prior guidelines for PCI, coronary artery bypass grafting (CABG) and ACS management that specifically addressed interval evidence published on the optimal duration of DAPT for various patient populations. Central to this update was the new recommendation for consideration of shorter-duration DAPT for patients at lower ischaemic risk with higher bleeding risk.23 The focused update also marked the first time official American guidelines sourced data examining performance of second-generation DES, which inherently carry a significantly lower risk of ST and as a result had effectively supplanted first-generation DES in modern practice.8,16,23
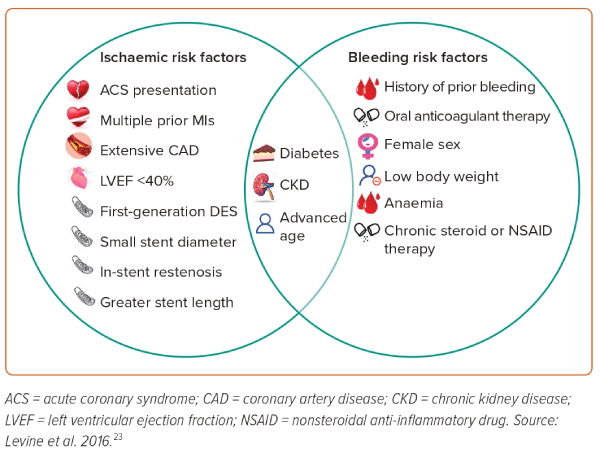
Assessment of a patient’s bleeding and ischaemic risk factors is integral to determination of optimal DAPT duration. Factors that may increase the risk of ISR or ischaemic events include advanced age, ACS presentation, extensive CAD history, diabetes and LVEF <40%; conversely, factors that may increase bleeding risk include a history of prior bleeding events, current anticoagulation therapy, female sex or chronic steroid/nonsteroidal anti-inflammatory drug therapy.23,25–28 A full list of ischaemic and bleeding risk factors adapted from the 2016 ACC/AHA focused update is illustrated in Figure 2.
Certain patients at a high risk of ischaemic events but low risk of bleeding events may be indicated for prolonged DAPT duration longer than 1 year. The authors of the Dual Antiplatelet Therapy Study developed a ‘DAPT score’ to stratify the benefit/risk ratio for patients based on the above-identified risk factors.29 The DAPT score takes into account the patient’s age, cigarette smoking status, presence of diabetes, history of MI and PCI, type and diameter of any stents and presence of heart failure. In patients treated for 1 year with DAPT without any significant adverse events, a DAPT score of ≥2 had a significant reduction in ischaemic events without a concomitant increase in bleeding risk with prolonged DAPT duration. In patients with a DAPT score of <2, the absolute risk increase in bleeding events was over two-times greater than the absolute risk reduction in MI or ST. The PRECISE-DAPT score is an iterative update that has been further validated by several trials to evaluate bleeding risk and guide DAPT duration in patients with ACS following PCI.30–32 However, prolonged DAPT, even in high-risk ACS patients, remains a class 2b recommendation per ACC/AHA guidelines and necessitates a nuanced risk/benefit consideration, as data suggest that extended DAPT for 18–36 months post-MI may decrease the rate of ischaemic complications by 1–3% but is accompanied by an absolute rate increase in bleeding complications of around 1%.3,33,34
In patients undergoing PCI with DES placement, the recommended duration of DAPT will primarily depend upon the patient presentation (stable CAD versus ACS) and bleeding risk. The ACC/AHA maintains a strong class 1 recommendation for at least 6 and 12 months of DAPT for stable CAD and ACS, respectively. However, in the case of high bleeding risk (e.g. recent major surgery or being on anticoagulation therapy), there is a class 2b recommendation to halve DAPT duration for each presentation to 3 and 6 months, respectively.23 This was in stark contrast to the 12-month minimum recommendation for all PCI patients treated with first-generation DES outlined in prior guidelines.35 This shift towards more conservative management was informed by several large randomised controlled trials and meta-analyses that did not find an increased risk of ST or ischaemic events in shorter DAPT duration (3–6 months) when compared with the previous 12-month standard.36–41
ESC 2017 Focused Update on DAPT in Coronary Artery Disease
In keeping with the ACC/AHA, the ESC released a focused update on DAPT duration in August 2017 to reflect new evidence that had surfaced in interval years.42 The message was largely the same as the update from their American counterparts, with few small differences that are discussed below and illustrated in Figure 3.
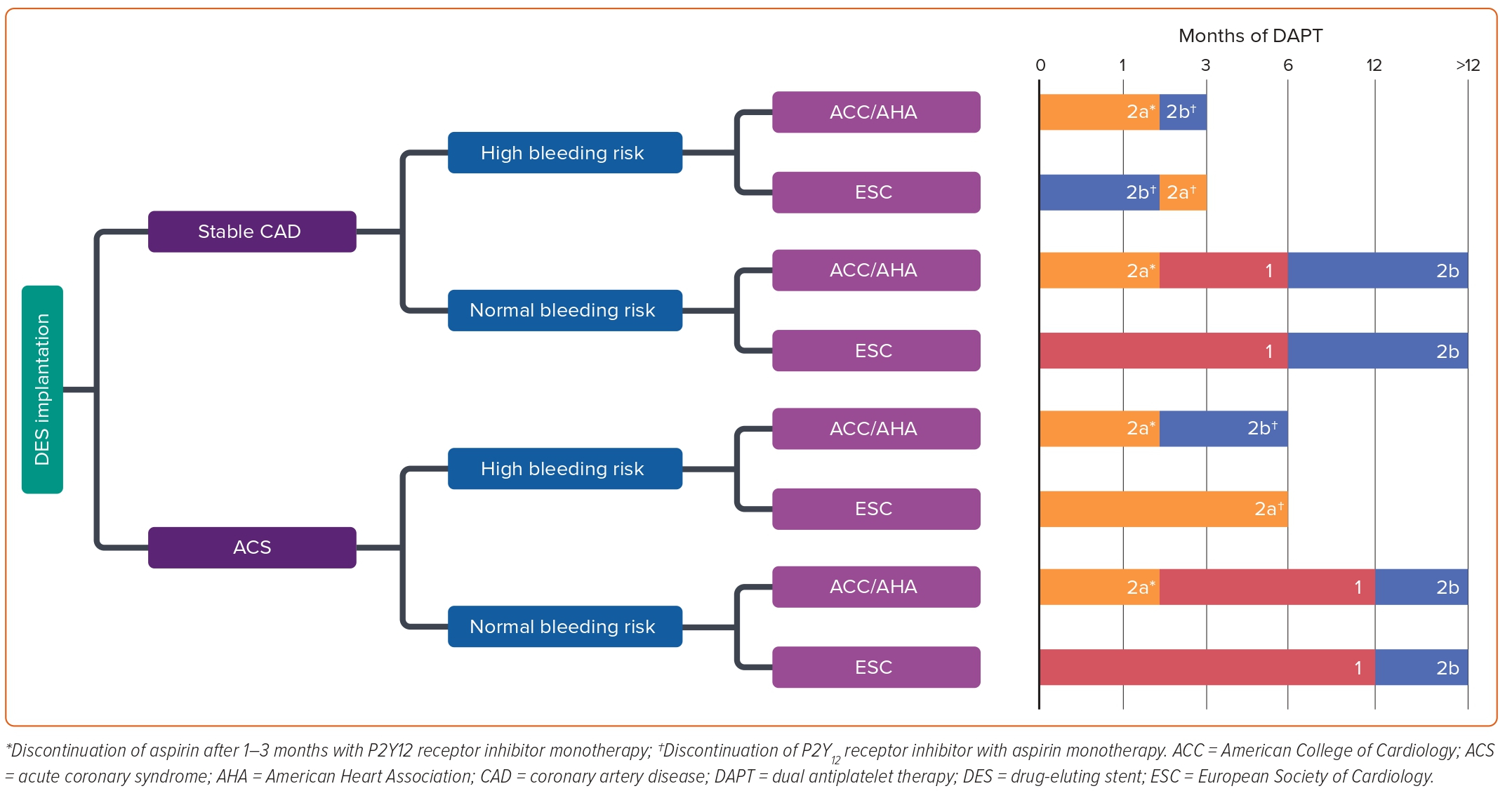
Like the ACC/AHA guidelines, the ESC framework also stratifies recommendations based upon patient presentation (stable CAD versus ACS) and bleeding risk. As with the ACC/AHA, there is a class 1 recommendation for 6- and 12-month DAPT for patients without high risk for bleeding who receive treatment for stable CAD and ACS, respectively. The ESC guidelines maintain a class 2b recommendation for extended DAPT >12 months only in patients with prior MI.42 This was predicated on a meta-analysis incorporating patients with previous MI from multiple large trials that concluded a significant reduction in each component of the primary endpoint including cardiovascular death, MI and stroke at the cost of significantly increased risk of major bleeding. However, all-cause mortality was noted to be the same between the two groups and the absolute risk reduction in cardiovascular mortality was found to be small at 0.3%.43 For patients with elevated bleeding risk, there is a class 2a recommendation for 3- and 6-month DAPT duration for stable CAD and ACS, respectively, in contrast to the class 2b recommendations put forth by the ACC/AHA. Much of the evidence base was shared between the two society guidelines; however the ESC update also included the RESET and OPTIMIZE trials, which both concluded that a 3-month DAPT duration did not result in significantly increased MACE compared with a 12-month DAPT duration.38,44
Notably, the ESC guidelines broke the standard by also including a class 2b consideration for 1-month DAPT duration in stable CAD patients with an unacceptably high bleeding risk (Figure 3).42 This recommendation was informed by two trials examining 1-month DAPT that concluded decreased risks of re-intervention, MI, and ST following implantation of the Endeavor Sprint stent (Medtronic) or BioFreedom drug-coated stent (Biosensors) compared with similar duration therapy for BMS implantation. However, these studies only included zotarolimus-eluting stents and did not compare outcomes for 1-month versus longer DAPT duration amongst second-generation DES, which raised concerns on their external validity.42,45,46
ACC/AHA/SCAI 2021 Guideline for Coronary Artery Revascularisation
In late 2021, the ACC/AHA/SCAI released an updated coronary artery revascularisation guideline meant to supplant all or part of numerous previous guidelines, including the 2011 PCI and CABG guidelines as well as the 2013 ST-elevation MI (STEMI) and 2014 non-STEMI-ACS guidelines to which the 2016 focused update to DAPT duration was applied.23,24 This update included further interval trials providing evidence for short-term 1- to 3-month DAPT and provided a new class IIa recommendation for discontinuation of DAPT after 1–3 months following stent implantation followed by P2Y12 receptor inhibitor monotherapy thereafter (Figure 3).24 Interestingly, this same class IIa recommendation was applied to PCI in both stable CAD and for ACS, even though the trials included in the 2021 guidelines rarely included patients with STEMI.47–51 The prior IIb recommendation for 3 months of DAPT followed by aspirin monotherapy in patients at high bleeding risk was maintained, and the authors noted a continued dearth of studies comparing outcomes of short-term DAPT followed by P2Y12 receptor inhibitor monotherapy versus aspirin monotherapy.
Overall Evidence and Recommendations
This section will provide an outline of general recommendations summarised from both most recent guidelines from ACC/AHA/SCAI and ESC regarding DAPT duration following DES implantation in various demographics, a visual representation of which may be found in Figure 3. Primary supporting evidence for guideline recommendations are provided alongside each recommendation.
In patients without increased bleeding risk, DAPT is recommended for 1 year in ACS and for 6 months in stable CAD, each followed by aspirin monotherapy indefinitely. In these patients who complete the recommended DAPT course without occurrence of major bleeding events, those who are at high risk of repeat adverse cardiovascular events (e.g. those with a history of previous MI) may be considered for continuation of DAPT for longer than 1 year. The evidence for shorter DAPT duration of 6 months for patients receiving PCI for stable CAD was based on two seminal trials from the early 2010s.
The EXCELLENT trial randomised 1,443 patients to either a 6-month DAPT (aspirin + clopidogrel) treatment arm or to a 1-year DAPT arm following PCI with DES implantation for stable CAD. The primary outcome was a composite of cardiac death, MI or ischaemia-driven target vessel revascularisation at 12 months and was demonstrated in 4.8% in the shortened DAPT group compared with 4.3% in the standard DAPT group (p=0.001 for noninferiority).37
The PRODIGY trial randomised 2,013 patients to groups receiving 6 months versus 24 months of DAPT (aspirin + clopidogrel) with a primary composite outcome consisting of all-cause mortality, MI, and stroke or cerebrovascular accident. There was no significant difference in incidence of the primary composite outcome at 24-month follow-up (10.1 versus 10.0%, p=0.91), but short DAPT was associated with a lower risk of major Bleeding Academic Research Consortium (BARC) bleeding events (1.9 versus 3.4%; HR 0.56; 95% CI [0.32–0.98]; p=0.037). Notably, subjects exhibited heterogeneity in clinical presentation, with approximately 75% of patients presenting with ACS while 25% had stable CAD. Analysis of net adverse clinical events (NACE) demonstrated increased incidence with extended DAPT in stable CAD (13.3 versus 5.6%; HR 2.5; 95% CI [1.35–4.69]; p=0.004) but not in ACS patients (16.1 versus 14.1%; HR 1.15; 95% CI [0.88–1.50]; p=0.29).52
Several other studies since 2014 have lent further credibility to the superiority of shortened DAPT, the largest of which was ISAR-SAFE, a double-blind randomised study including 4,005 patients, 60% of whom presented with stable CAD and 40% with ACS. The study compared 6-month DAPT (aspirin + clopidogrel) with 12-month DAPT and found there was no difference in the incidence of the primary composite endpoint of death, MI, ST, stroke and major bleeding (1.5 versus 1.6%; p<0.001 for noninferiority) in both ACS and stable CAD patients.39 The ITALIC and SECURITY trials demonstrated similar outcomes comparing 6-month DAPT with 12-month or 24-month DAPT. 36,53
In patients with elevated bleeding risk presenting with stable CAD, DAPT should be continued for 3 months followed by aspirin monotherapy. However, those at unacceptably high risk of bleeding may be considered for transition to aspirin monotherapy beginning at 1 month. For high bleeding risk patients presenting with ACS, DAPT should be continued for 6 months followed by aspirin monotherapy. Alternatively, high bleeding risk patients with either presentation may be considered for 1–3 months of DAPT, followed by indefinite P2Y12 receptor inhibitor monotherapy.
The initial studies providing strong evidence for a shorter 3-month DAPT duration came in the form of the RESET and OPTIMIZE trials. RESET randomised 2,117 patients to 3- and 12-month DAPT (aspirin + clopidogrel) and did not find any difference in the primary composite endpoint of all-cause mortality, MI or ST (0.8 versus 1.3%; p=0.48).44 Of the subjects included, 28% presented with ACS. A subgroup analysis of ACS-presenting patients showed a trend toward an increased incidence of the primary composite endpoint in patients randomised to the short DAPT group but did not reach statistical significance (p=0.158). OPTIMIZE randomised 3,119 patients also to 3- and 12-month DAPT with aspirin + clopidogrel and produced similar outcomes, with the primary endpoint of NACE occurring in 6.0% of the short DAPT group and 5.8% in the long DAPT group (p=0.002 for noninferiority).38 Notably, high-risk ACS and MI patients were excluded from the analysis.
More evidence has emerged in recent years corroborating the efficacy and safety of 3-month DAPT duration with P2Y12 receptor inhibitor monotherapy thereafter. Mehran et al.47 and the TICO trial compared 3 month with 12-month DAPT with aspirin and ticagrelor followed by ticagrelor monotherapy.50 Mehran et al. demonstrated no significant difference in NACE (3.9 versus 3.9%; HR 0.99; 95% CI [0.78–1.25]; p<0.001 for noninferiority) while the TICO trial found that short DAPT decreased incidence of NACE (3.9 versus 5.9%; HR 0.66; 95% CI [0.48–0.92]; p=0.01). Both showed a significant reduction in major BARC bleeding in the short DAPT group (Mehran et al., 4.0 versus 7.1%; HR 0.56; 95% CI [0.45–0.68]; p<0.001, and TICO, 1.7 versus 3.0%; HR 0.66; 95% CI [0.48–0.92]; p=0.01). The SMART-CHOICE trial randomised 2,994 patients to 3- and 12-month DAPT with aspirin and a P2Y12 receptor inhibitor followed by aspirin discontinuation and P2Y12 receptor inhibitor monotherapy and demonstrated similar results, showing no significant difference in NACE (4.5 versus 5.6%; HR 0.81; 95% CI [0.58–1.12]; p=0.20) and a significant reduction in major bleeding events (2.0 versus 3.4%; HR 0.58; 95% CI [0.36–0.92]; p=0.02).48
In patients with AF on anticoagulation, both modern ACC/AHA and ESC guidelines recommend a short duration of triple therapy with DAPT and non-vitamin K antagonist oral anticoagulant (NOAC) [ACC/AHA recommends 1–4 weeks while ESC recommends up to 1 week or up to 1 month with high ischaemic risk] followed by discontinuation of aspirin.24,54 The ESC further allows for discontinuation of the P2Y12 receptor inhibitor after 6 months for NOAC monotherapy thereafter for patients deemed at high bleeding risk. These guidelines incorporate the AUGUSTUS trial, which randomised patients with AF who presented with ACS and underwent PCI with DES implantation to receive a P2Y12 receptor inhibitor with either apixaban or warfarin and with either aspirin (triple therapy) or placebo. The trial concluded that NOAC compared with warfarin was associated with a significantly lower risk of major bleeding events (10.5 versus 14.7%; HR 0.69; 95% CI [0.58–0.81]; p<0.001) as well as a lower incidence of death or hospitalisation (23.5 versus 27.4%; HR 0.83; 95% CI [0.74–0.93]; p=0.002). Triple therapy with aspirin compared with placebo was also associated with higher incidence of bleeding (16.1 versus 9.0%; HR 1.89; 95% CI [1.59–2.24]; p<0.001) without any difference in death or hospitalisation rates.55
New Evidence on the Feasibility of 1-month DAPT for Select Patients
In the years since the focused updates from the American and European cardiology societies, several studies have been published that provide evidence for even more conservative 1-month DAPT management following PCI in certain patient populations.56 Consideration of this ultra-short duration of DAPT is important for patients with unacceptably high bleeding risk (e.g. those with AF requiring long-term anticoagulation) or for those who require urgent to semi-urgent surgical intervention for other comorbid conditions, as in the patient case presented at the beginning of this review.
Although the newest 2021 guidelines released by the ACC/AHA/SCAI incorporate many of the highest-profile studies in this space, questions remain on the exact protocol in transitioning from DAPT to anti-platelet monotherapy, especially in comparing the efficacy of P2Y12 receptor inhibitor versus aspirin monotherapy.
Furthermore, the emergence of personalised medicine and genomics-guided therapy may further necessitate consideration of minimal DAPT duration for certain patients, as evidenced by the ‘East Asian paradox’ that has been described as a propensity in East Asian patients for a lower rate of ischaemic events but higher rate of bleeding events after PCI.57 Indeed, the accumulation of data suggesting significant heterogeneity among patients in clinical resistance to antiplatelet therapy points to the evolving nature of situational considerations for DAPT, although to date there is a lack of large randomised trials demonstrating benefit of personalised antiplatelet therapy and no society guideline recommends routine platelet-function testing in clinical practice.35,42,58–60
The gradual accumulation of studies examining shorter durations of DAPT lends additional credence to the ESC’s unique recommendation for 1-month DAPT duration in select stable CAD patients and the 2021 ACC/AHA/SCAI recommendations for 1- to 3-month DAPT for all patients receiving DES implantation.
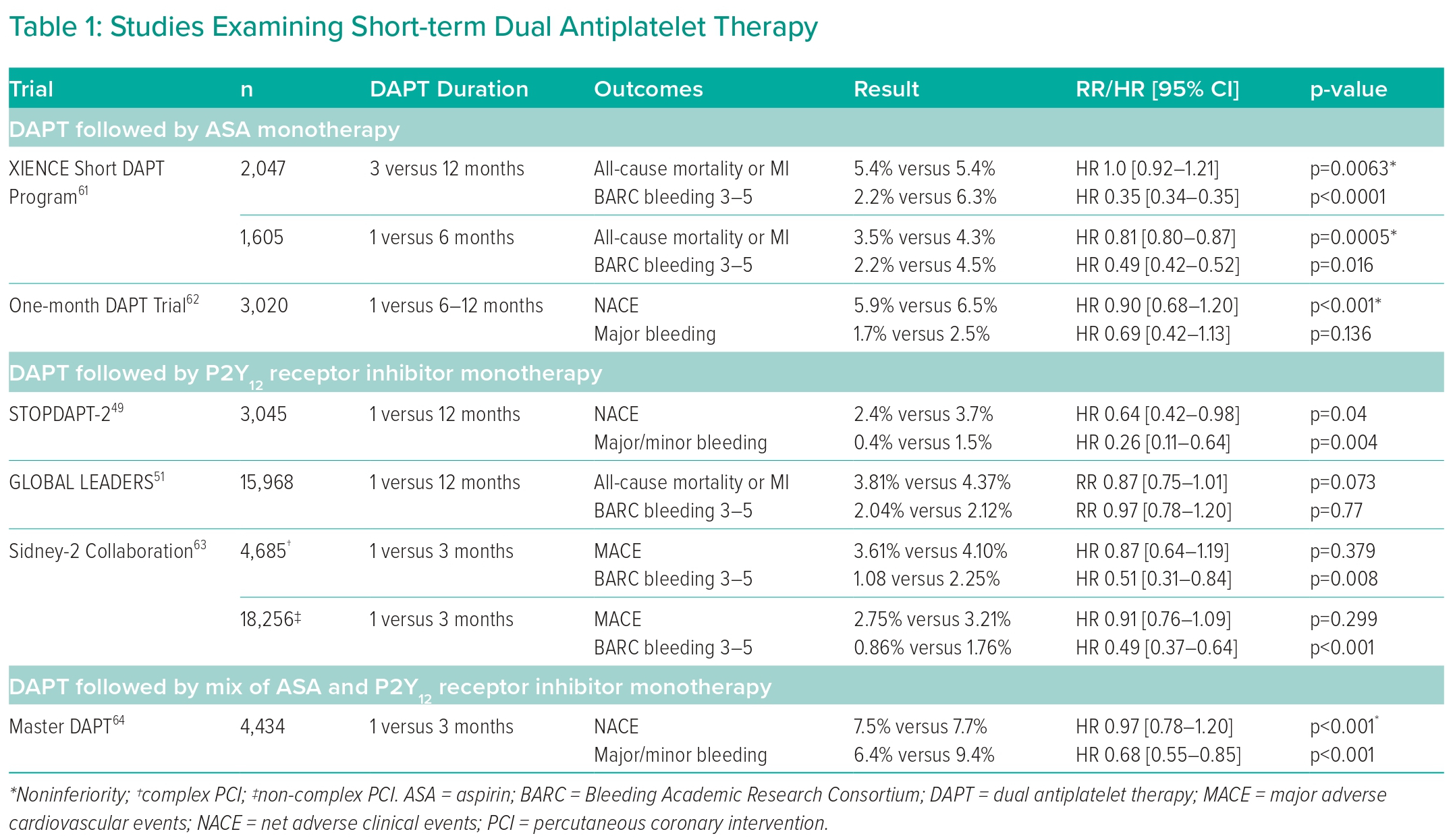
The following section will elaborate upon recent studies examining short-term DAPT duration following PCI with DES implantation; Table 1 shows a full list of notable trials and their findings. In general, all studies examined 1- or 3-month DAPT compared to 6–12-month DAPT following PCI using a variety of different stent models and cardiac presentations. Outcomes were largely uniform across multiple trials, demonstrating noninferiority of 1- or 3-month DAPT compared with 6–12-month DAPT in terms of preventing post-PCI adverse MACE, although the TICO randomised clinical trial is notably absent in this characteristic and, in fact, found short-term DAPT to improve outcomes.47–51,61,62 The noninferiority of short-term DAPT compared with standard DAPT persisted even in patients undergoing complex PCI procedures.63
Most studies also demonstrated a decreased rate of adverse bleeding events with short-term DAPT, but this finding was not exhibited by the One-month DAPT trial and the GLOBAL LEADERS trial.51,62
Of note, while many trials have examined short-term DAPT with duration lasting from 1 to 3 months, the only trial to directly compare 1- to 3-month DAPT was the MASTER-DAPT trial, which found 1-month DAPT to be noninferior to 3-month DAPT with relation to NACE and MACE while also offering a statistically significant reduction in postoperative bleeding risk.64 There is a paucity of other trials directly comparing these two short-term DAPT protocols, making this one arena in which future research may be applied to further optimise post-PCI DAPT management. The 2021 ACC/AHA/SCAI guidelines reflect this uncertainty with its nonspecific 1–3-month DAPT recommendation.24
The studies included here incorporated a mix of aspirin and P2Y12 receptor inhibitor monotherapy following cessation of DAPT. The majority of trials were designed to continue patients on P2Y12 receptor inhibitor monotherapy following a prespecified DAPT duration, but the One-month DAPT trial and the XIENCE Short DAPT Program both demonstrated similar clinical benefits in short-term DAPT followed by aspirin alone.61,62 However, there is emerging evidence that P2Y12 receptor inhibitor monotherapy may be superior to aspirin monotherapy in reducing both postoperative cardiovascular and cerebrovascular events and major bleeding events.65,66
The HOST-EXAM trial was a prospective, randomised, multi-centre trial in South Korea that compared aspirin with clopidogrel monotherapy in patients following 6–18-month DAPT. At 24-month follow-up, clopidogrel monotherapy was associated with significantly lower composite risk of all-cause death, nonfatal MI, stroke and readmission for ACS as well as significantly reduced risk of bleeding events.67 Similar benefits have been reported for P2Y12 receptor inhibitor monotherapy in the secondary prevention of MACE in the absence of PCI with DES implantation, although the benefit there may be marginal with a high number needed to treat.68 The 2021 ACC/AHA/SCAI guidelines also reflect the evolving state of research by maintaining separate weak and moderate recommendations for aspirin and P2Y12 receptor inhibitor monotherapy following DAPT, respectively (Figure 3).24
In patients at high bleeding risk, consideration should also be made towards the use of drug-coated balloons (DCB) if appropriate. The recommended duration of DAPT following a DCB revascularisation strategy is reported by the 2017 ESC focused update to be 3–12 months; however recent consensus reports have revised optimal duration to be 4 weeks. 42,69 A comprehensive review by the International DCB Consensus Group found DCBs to be non-inferior to second-generation DES in treating small vessel (<3.0 mm) lesions. Several trials have demonstrated the noninferiority of DCBs compared with DES even in ACS patients, although there is limited evidence for DCBs in treatment of larger vessel lesions. For patients at exceptionally high risk of bleeding, some have even suggested that DAPT may be omitted entirely following a DCB strategy, which may make this approach preferable over 1-month DAPT following PCI with DES for certain high-risk patients.70–72 However, it should be noted that DCBs are not recommended over DES for coronary revascularisation per the latest AHA/ACC/SCAI guidelines.24
Case Revisited
Upon further review of the literature presented and discussed above, we determined it appropriate to proceed with left heart catheterisation and PCI with DES implantation if indicated, followed by DAPT for 1–3 months as clinically appropriate to expedite the nephrectomy. Mr Q was found to have 60% stenosis of the right coronary artery and an 80% type A lesion of the left anterior descending (LAD) artery. A 3.5 × 28 mm DES was successfully placed in the LAD without complications. He was initiated on maximal guideline-directed medical therapy for heart failure with reduced ejection fraction, and 1 month follow-up demonstrated interval improvement of ejection fraction to 25%. The patient was continued on DAPT with aspirin and ticagrelor for 1 month, after which aspirin was discontinued and he was continued on ticagrelor monotherapy. He subsequently underwent successful nephrectomy with urology.
Conclusion
Discussions on the optimal duration of DAPT have shifted dramatically in the last decade from rigid 6–12-month recommendations to a more flexible and dynamic protocol that accounts for various demographic factors including the state of CAD and bleeding risk. Emerging evidence has provided strong support for short-term DAPT of 1–3 months in select patients, and societal guidelines are gradually being updated to reflect this new status quo. However, more research is needed to compare the efficacy and safety of 1- versus 3-month DAPT and of post-DAPT aspirin versus P2Y12 receptor inhibitor monotherapy. Future work may also provide stronger data for the indications of long-term DAPT of longer than 12 months for low-risk patients.