Refractory angina is a major global cardiovascular healthcare challenge. Patients experience chronic debilitating symptoms that significantly impact on their morbidity, mental health, and quality of life. As the population ages and survival from acute coronary syndromes improves, the burden of chronic coronary syndromes is increasing with a concomitant rise in the prevalence of patients with refractory angina encountered in clinical practice. Currently, treatment options are limited, and new effective therapies are urgently needed to improve patient outcomes.1–3 One emerging treatment option that is attracting increasing attention is the coronary sinus reducer (CSR).
In this article, we will review the unmet clinical needs of patients with refractory angina, describe the development of the CSR device and critically review the evidence for its mechanism of action and clinical effectiveness, highlighting knowledge gaps and areas of on-going research.
Healthcare Burden of Refractory Angina
Refractory angina is conventionally defined when symptoms due to coronary insufficiency in the presence of coronary artery disease (CAD) persist for ≥3 months despite a combination of optimal medical therapy, angioplasty or coronary artery bypass grafting (CABG). Reversible myocardial ischaemia should be demonstrated.4
The epidemiology of refractory angina remains suboptimally described and contemporary data are needed. However, 5–15% of patients with a diagnosis of angina progress to becoming refractory.1,4 In a Swedish survey of patients with severe angina, 9.6% of patients referred for revascularisation were considered unsuitable.5 In Europe, the annual incidence of refractory angina is estimated at 30,000–50,000 new cases per year.4,6 In Canada, around half a million patients are living with refractory angina; in the US this is estimated between approximately 600,000 and 1.8 million patients, with approximately 75,000 new cases per year.7,8 Specific data for the UK are lacking. By applying the published rate of 6.7% of patients undergoing angiography considered unsuitable for further revascularisation to the number of angiograms performed in England it can be estimated that approximately 15,000 new cases of refractory angina occur in England per year.2,9 The recent provision of a specific diagnosis code (I20.2) for refractory angina in ICD-10-CM will not only aid clinician recognition of this condition but also improve data collection for studies of epidemiology, clinical outcomes and health economic burden.
Mortality from refractory angina has been evaluated in the prospective OPTIMIST registry, which included 1,200 patients with a (mean age 63.5 years), with high rates of triple-vessel CAD (78.3%) and previous revascularisation (CABG 72.4%, percutaneous coronary intervention [PCI] 74.4%).10 Mortality at 1-year was 3.9% and 28.4% at 9 years, which was lower than previously reported.10–13 Therapeutic goals for this group of patients should, therefore, predominantly focus on symptom relief and improving quality of life. Management strategies that address these unmet needs may also reduce healthcare costs associated with refractory angina, which are known to be high (approximately US$10,185 per patient over a 3-year period) driven by frequent hospitalisations and repeated investigations that often do not result in major alterations in treatment.
Clinical Descriptors of Patients with Refractory Angina Secondary to Coronary Artery Disease
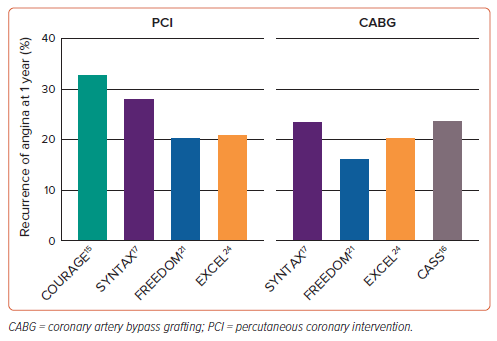
In patients with refractory angina, reasons for failure of medical therapy include inadequate or inappropriate empirical pharmacological treatment, intolerance or poor adherence. Revascularisation may not be suitable because of unsuitable coronary anatomy, previous percutaneous or surgical revascularisation with no further targets for intervention, a lack of graft conduits or concurrent medical co-morbidities that incur prohibitively high procedural risk.14 Contemporary data also suggest that many patients experience residual chest pain after revascularisation (Figure 1).15–18 In the COURAGE trial, 34% of patients after PCI and 42% of patients on medical therapy reported recurrent angina at 1 year.15 By 3 years, this was 28% and 33%; and at 5-year follow-up, 26% and 28%, respectively. Other studies have reported similar prevalence rates of persistent symptoms after revascularisation.19,20
The problem of recurrent post-revascularisation angina is also well described in patients undergoing surgical revascularisation. Data from the CASS registry showed 24% of patients after CABG to have a recurrence of their angina within the first year. By 6-year follow-up, this had increased to 40%.16 The results of contemporary trials of PCI versus CABG for left main and multivessel CAD show this issue is still highly prevalent. In the SYNTAX trial of 1,428 patients, residual angina at 1 year was common after either PCI (28.3%) or CABG (23.7%).17 Similar rates were also observed in the 1-year results of the FREEDOM trial (PCI 20.5%; CABG 16.5%) and EXCEL trials (PCI 21.1%; CABG 20.5%).18,21–24
The pathophysiology of recurrent angina after revascularisation is multifactorial and may include both structural and functional disorders of the coronary circulation. Abnormalities may exist in the epicardial coronary arteries, such as flow-limiting epicardial obstructions due to stent or bypass failure, progression of CAD, incomplete revascularisation, myocardial bridge or diffuse epicardial disease. Functional disorders of the coronary circulation, such as vasospasm (which can be epicardial and/or microvascular) and coronary microvascular dysfunction, may also occur.25,26 Importantly, these mechanisms may co-exist. Diagnostic methods that consider the responsible mechanism(s) must be employed, allowing stratified therapy depending on the underlying pathophysiology. Studies have suggested comprehensive investigation with invasive coronary physiology as an effective strategy that allows tailored therapy and improves symptoms and quality of life.27 However, few effective medical or percutaneous therapies exist, particularly for patients with diffuse epicardial atherosclerotic disease and coronary microvascular dysfunction (CMD), who continue to experience greatly impaired quality of life, supporting the need for new evidence-based treatments.25,26,28–30
Coronary Sinus Reducer: Historical Context and Development
The coronary sinus reducer (CSR; Shockwave Medical) is an hourglass-shaped stainless-steel device inserted percutaneously through the jugular vein into the coronary sinus to treat refractory angina. The device becomes endothelialised over several months and creates a controlled narrowing in the coronary sinus that raises venous backpressure, dilates arterioles in the ischaemic subendocardium and improves myocardial perfusion. There is growing interest in this interventional therapy for patients with refractory angina.31
The principle of coronary sinus reduction to improve myocardial perfusion and angina was first tested by Claude Beck in Cleveland, Ohio, in the 1930s prior to the advent of cardiopulmonary bypass. 32,33 After extensive preclinical development, this approach was translated to patients using a procedure involving a sternotomy and partial ligation of the coronary sinus to create a stenosis with a 3 mm diameter lumen. Additionally, mechanical epicardial abrasion and application of asbestos to the epicardial surface of the heart was performed to induce inflammation, stimulate collateral vessel development and promote neovascularisation.34 It has been hypothesised that these effects may have also promoted the secretion of paracrine angiogenic factors from mediastinal fat.34 In preclinical work, Beck found that these operations increased collateral blood supply to ischaemic myocardium beyond a ligated circumflex artery and were associated with reduced infarct size, improved myocardial contractility and reduced mortality.33 In a series of over 600 patients, after an inpatient stay of approximately 2 weeks, 90% of patients experienced angina relief and were able to return to work, although detailed follow-up is not available. Improved 5-year mortality was observed.33
Inspired by this work, Sheinfeld, Paz and Tsehori designed a percutaneous device to emulate Beck’s surgical procedure.36 The CSR device is balloon-expandable and pre-mounted on its own delivery system. When inflated to a pressure of 4 atmospheres, the proximal and distal diameters are 13 mm and 9 mm respectively. The waist of the hourglass shape remains constant at 3 mm up to an inflation pressure of 8 atm. The minimal diameter at the neck of the CSR is matched to the residual lumen diameter after partial coronary ligation in Beck’s surgical procedure. Beyond this inflation pressure, the balloon takes on a tubular shape with loss of the waist.36
In preclinical studies, the CSR has been shown to endothelialise and promote tissue ingrowth to create the narrowing and develop its haemodynamic effect in the coronary sinus.37 In a first-in-human study, Banai et al. implanted the CSR device in 15 patients with refractory angina due to advanced CAD. All procedures were completed successfully with no procedure-related adverse events. Angina improved in 86% of patients (mean baseline Canadian Cardiovascular Society [CCS] score 3.07, which improved to 1.64 at follow-up; p<0.0001). Stress-induced ST-segment depression improved in six of nine patients and the extent and severity of ischaemia assessed by dobutamine stress echocardiography (DSE) and thallium single-photon emission CT were also significantly improved.36
Current Evidence for Clinical Efficacy and Safety
The randomised, double-blinded, sham-controlled COSIRA trial recruited 104 participants with refractory angina due to advanced CAD who were randomised 1:1 either to CSR implantation or a sham procedure.31 The study met its primary outcome, showing a significant difference in symptom severity adjudicated using CCS class. Compared with 15% of patients in the sham group (eight of 52), 35% of patients (18 of 52) improved by ³≥2 CCS classes after CSR implantation (p=0.02). Furthermore, a significant proportion of patients improved by ³≥1 CCS class (71% CSR arm versus 42% in sham arm; p=0.003). Quality of life assessed using the Seattle Angina Questionnaire (SAQ) also significantly improved after CSR (17.6 points) compared with sham (7.6 points; p=0.03). However, no significant change in total exercise duration at 6 months was observed (CSR: 59 seconds, 3%; sham: 4 seconds, 1%; p=0.07).
Additionally, no significant difference in wall-motion score index assessed by DSE was observed (CSR: 14% versus sham: 8%; p=0.20), although there was a trend to improvement when stratified by the left coronary artery distribution (CSR: 13% versus sham: 3%; p=0.06). Importantly, CSR implantation was shown to be safe. Out of 34 serious adverse events, 10 occurred in the CSR arm and 24 occurred after sham. In a post hoc efficacy analysis of patients with CCS class 3–4 angina, symptoms, functionality, and quality of life improved concordantly after CSR implantation compared with sham.38
These initial results have been supported by real world registry data. The RESOURCE study was a retrospective, observational, single arm ‘real-world’ registry that included 658 consecutive patients with refractory angina undergoing CSR implantation across 20 high-volume centres in Europe, UK and Israel.39 Clinical efficacy was similar to that observed in the CSR arm of the COSIRA trial. At a median follow-up of 502 days, 39.7% of patients improved by ³≥2 CCS classes and 76% by ³≥1 class. Procedural success was high (96.7%) and complication rates were low (5.7%; 38 of 663 attempted procedures). No bailout surgery, intra- or peri-procedural death or MI were reported. The most frequent complications were device or delivery catheter-related, such as device embolisation and migration. Severe complications such as coronary sinus dissection (nine of 42 complications) or perforation (three of 42 complications) were rare and managed conservatively or with minimally invasive approaches. Several smaller national registries from Israel, Italy, Belgium, the Netherlands and Poland have demonstrated similar results.40–45
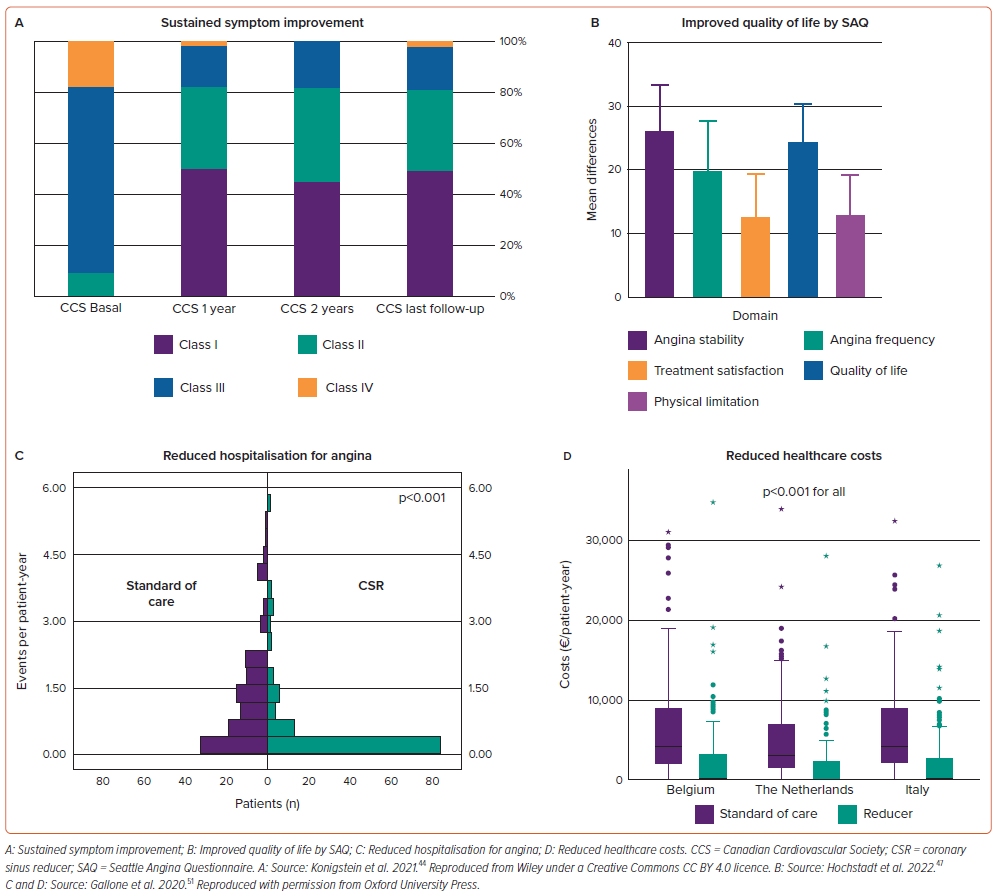
The prospective multicentre observational REDUCER-1 study has completed recruitment of 400 patients and is due to report in 2024. This registry enrolled patients undergoing CSR implantation in centres in Europe. Interim results of the first 228 patients have shown clinical effectiveness of CSR implantation consistent with other published studies.46 Furthermore, a sustained symptom benefit over 2 years was also demonstrated (Figure 2A). Baseline CCS class was 2.8 ± 0.6, which improved to 1.8 ± 0.7 at 2 years. While 70% of patients had a baseline CCS class of 3–4, this reduced to 15% at 2-year follow-up. Symptom improvements were associated with improved functional class and quality of life; implant success was high (99%) with no safety concerns.
These results have been summarised in a publication-level meta-analysis evaluating a primary outcome of the proportion of patients improving ³1 CCS class after CSR implantation.47 Nine studies (n=846) were included, with 76% of patients improving by ³1 CCS class and 40% improving ³2 CCS classes. Post procedural SAQ scores (Figure 2B) and distance achieved on 6-minute walk test also significantly improved. Procedural success was high (98%) with no major and few (3%) non-major periprocedural complications.
The recent double-blinded, sham-controlled ORBITA-COSMIC study confirms the angina improvement after CSR implantation previously demonstrated in the COSIRA trial.46 Daily angina episodes were improved after CSR compared with sham (OR 1.40; 95% CI [1.08–1.83]; probability of benefit=99.4%), with angina improvement occurring approximately 10 weeks after device implantation. However, this study did not meet its primary mechanistic endpoint of increased transmural stress myocardial blood by cardiac MRI (CMR). There was a signal of improved endocardial blood flow in visually ischaemic segments, though these results should be considered hypothesis generating.
An important consideration in the efficacy of CSR implantation is the durability of angina improvement. Over a median follow-up of 3.38 years, Konigstein et al. showed sustained symptom improvement in a study of 99 patients with refractory angina undergoing CSR implantation. Mean baseline CCS class was 3.1, which improved to 1.66 at 1 year (p<0.001), 1.72 at 2 years and 1.71 (p>0.05 compared with CCS class at 1 year) at last follow-up (median 3.38 years [interquartile range 2.95–4.40]).44 While 91% of patients experienced CCS class 3–4 angina at baseline, this reduced to 17.9% (p<0.001) at 1 year and 19% at last follow-up. During follow-up, 15.1% died, 9% had a MI and 21% underwent further PCI. Upcoming results from the REDUCER-1 registry will provide additional data on durability of angina improvement.
The paucity of effective therapies for refractory angina is reflected in the latest guidelines on chronic coronary syndromes from the European Society of Cardiology, where few therapies are recommended.49 Of note, CSR implantation has been given a class IIb, level of evidence B recommendation, such that this intervention may be considered for patients with debilitating anginal symptoms who have exhausted all options for medical therapy and mechanical revascularisation. Furthermore, a recommendation was provided by the National Institute for Health and Care Excellence in the UK in 2021 for CSR implantation in patients with refractory angina to provide symptomatic relief, reduce the need for anginal medications and improve quality of life.50
While evidence for the cost-effectiveness of CSR implantation is limited, Gallone et al. provided an initial report in 215 patients undergoing CSR implantation in the Netherlands, Belgium and Italy.51 A significant reduction in healthcare costs after CSR implantation was observed. This was due to a reduction in angina-driven hospitalisations, outpatient visits, coronary angiography and angioplasty. Reduced costs were observed across a range of European healthcare systems (Figure 2C and D). CSR implantation was associated with higher quality-adjusted life years (0.665 versus 0.580; p<0.001) and in the 1-year timeframe analysed, was assessed to be cost-effective.
Evidence for the Mechanism of Action of the Coronary Sinus Reducer
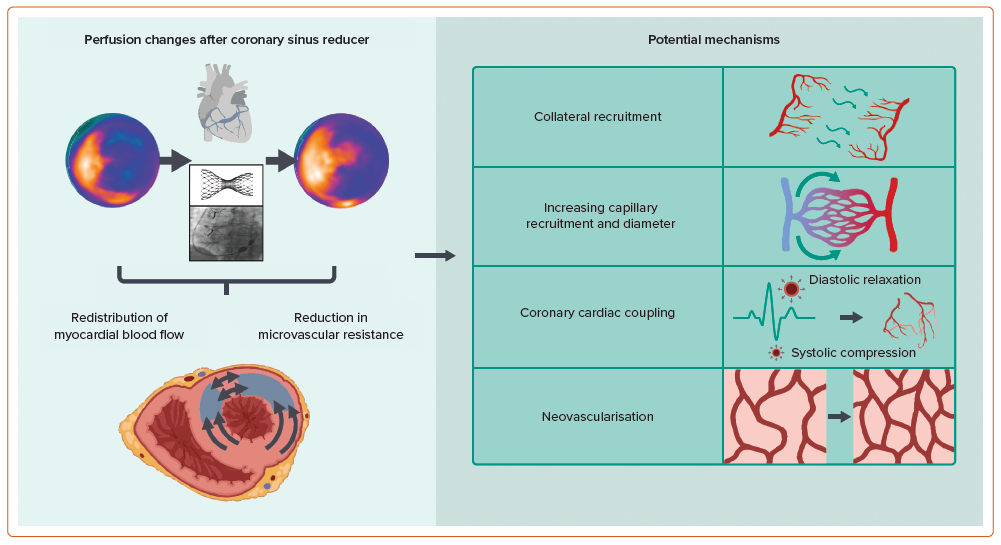
In the normal heart, subendocardial blood flow is higher than in the subepicardium.52–57 This is to meet a greater metabolic demand in the subendocardium due to increased work and contractility compared with the subepicardium. Intramyocardial pressure also varies across myocardial layers, being greater in the subendocardium compared with the subepicardium, and leading to differential compression of the microcirculation which may affect perfusion.58–60 Under normal physiological conditions, autoregulatory mechanisms maintain perfusion across a range of perfusion pressures. However, the subendocardium remains particularly susceptible to ischaemia, such as in the presence of epicardial coronary stenosis, and is associated with an impaired endocardial:epicardial blood flow ratio, particularly under circumstances of increased myocardial demand.61-66 The mechanism by which the CSR improves angina by altering myocardial perfusion remains to be determined. It has been hypothesised that by creating a narrowing in the coronary sinus and raising venous backpressure into the myocardium, subendocardial blood flow is enhanced and the endocardial:epicardial ratio is returned closer towards normal physiology with augmented collateral flow into ischaemic territories.67
This proposed mechanism of action has mainly been based on evidence from a canine preclinical experiment.68 In this study, the left anterior descending (LAD) artery was occluded either with or without concurrent coronary sinus occlusion. Coronary sinus occlusion increased blood flow in ischaemic myocardium, particularly the subendocardium (0.17 to 0.33 ml/min/g; p<0.05) and reversed the impaired endocardial:epicardial ratio (from 0.59 to 1.15; p<0.01).
In another canine study, Sato et al. showed that elevated coronary sinus pressure significantly improved regional blood flow after LAD occlusion, suggesting that this occurred through augmenting collateral flow.69 The presence of collateral channels has been suggested to be an important mediator by which elevations in coronary sinus pressure redistribute myocardial perfusion.34,70–72
However, extrapolation of these preclinical studies to inform the mechanism of clinical CSR implantation is challenging. In particular, acute coronary sinus occlusion, rather than chronic graded narrowing, has been the predominant experimental intervention used to increase coronary sinus venous pressure. Furthermore, experimental models of acute ischaemia by complete coronary ligation rather than chronic ischaemia have been used.
To overcome some of these difficulties, the CSR device was tested in a small preclinical study that induced chronic ischaemia in mini pigs.37 This model was chosen as the porcine ischaemic heart is similar to that of humans, with limited innate collateral circulation compared with dogs. Ischaemia was induced by surgical placement of an ameroid ring around the origin of the circumflex artery that created an occlusion confirmed by follow-up coronary angiogram at 6 weeks. In mini pigs that developed ischaemia confirmed by dobutamine stress echocardiography, CSR implantation (n=4) was associated with improvements in left ventricular contractility and myocardial perfusion (defined as a >50% reduction in area of ischaemic territory) at 6 weeks and 6 months follow-up.37
Mechanistic studies in humans are needed. Preliminary open-label studies without control arms measuring semi-quantitative metrics of stress perfusion by CMR have demonstrated improvements in myocardial perfusion and a reduction in ischaemic burden after CSR implantation.73,74 The number of segments with inducible perfusion abnormalities reduced from 92/240 (38%) at baseline to 69/240 (29%; p<0.001). In addition, there were significant improvements in transmural myocardial perfusion reserve index (MPRI; change (∆) in MPRI=0.355 in segments with baseline MPRI <1.3; ∆MPRI = −0.036 in segments with baseline MPRI 31.3; p<0.001). Greater improvements were observed particularly in the subendocardium of ischaemic segments, which was further supported by the results of ORBITA-COSMIC.48,73
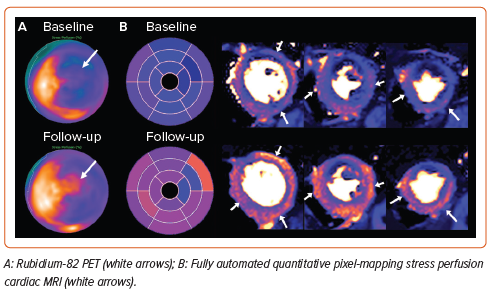
Recent reports have investigated changes in quantitative myocardial perfusion after CSR implantation using fully automated stress perfusion CMR and rubidium-82 PET have demonstrated significant improvements in myocardial blood flow, particularly towards the most hypoperfused segments.75,76 These results are further supported by in silico modelling studies.77 In summary, an increasing body of evidence suggests the potential of CSR implantation to improve myocardial ischaemia in patients with advanced epicardial coronary artery disease. Further insights will be provided by the results of the PET sub-study of the COSIRA-II clinical trial (NCT05102019), which is currently recruiting.
Several exploratory pilot studies using CMR and echocardiography have also reported improvements in left-ventricular systolic and diastolic function, strain and right-ventricular systolic function.78–80 Improvements in exercise capacity and oxygen kinetics by cardiopulmonary exercise testing (CPET) have also been suggested after CSR implantation. In a multicentre prospective study (n=37), patients underwent CPET before CSR implantation and at 6-month follow-up.81 Significant improvements in VO2 max (+11.3%; p=0.026) and workload (+12.9%; p=0.05) were observed but not in VO2 at the anaerobic threshold. Recently, a small randomised, sham-controlled study (n=25) demonstrated an increase in VO2 max from 15.56 ± 4.05 to 18.4 ± 5.2 ml/kg/min (+18.3%; p=0.03) after CSR implantation but no change in the sham group (p=0.53) at 6 months follow-up.82 Interestingly, no differences were observed in CCS class or SAQ scores between groups.
Recent evidence has also suggested that the CSR exerts direct effects on the coronary microcirculation. In patients with angina and unobstructed coronary arteries, CSR implantation increased coronary flow reserve (CFR) and reduced index of microvascular resistance (IMR) at 6 months assessed by bolus thermodilution.83,84 The hypothesis that increasing coronary venous pressure would alter microvascular resistance was tested in patients with CMD.85 In a blinded, sham-controlled, crossover, randomised clinical trial, 20 patients were recruited with moderate-severe angina (CCS class 2–4) and evidence of CMD (IMR >25). Inflation of an undersized balloon in the coronary sinus resulted in an increase in coronary sinus venous pressure at rest and during hyperaemia (300% and 317%, respectively, compared with sham; p<0.001). A decrease in hyperaemic distal coronary pressure was observed together with a reduction in IMR (balloon: 14 [IQR: 8–26] versus sham: 31 [23–53]; p<0.001).
The recently published INROAD study evaluated 24 patients with refractory angina due to advanced obstructive CAD and previous coronary revascularisation and demonstrated reductions in IMR (baseline: 33.35 ± 19.88; 4-month follow-up: 15.42 ± 11.36; mean difference: −17.90; 95% CI [−26.16 to −9.64]; p<0.001) in 24 patients with refractory angina due to advanced obstructive CAD and previous coronary revascularisation. A significant improvement in CFR was also observed (baseline: 2.46 ± 1.52; 4-month follow-up: 4.20 ± 2.52; mean difference: 1.73; 95% CI [0.51–2.96]; p=0.007).86
The preliminary favourable results of paired open-label assessment of invasive microcirculatory assessment at baseline and 4–6 months after CSR implantation in patients with reduced coronary or acetylcholine flow reserve have been reported.87 Collectively, these studies suggest a potential effect of CSR implantation on coronary microcirculatory function. Several plausible mechanisms that may explain changes in myocardial perfusion and microvascular resistance include collateral recruitment, increased capillary recruitment and diameter, improved coronary cardiac coupling and neovascularisation, and are under investigation (Figure 3).
Finally, it has been reported that 15–30% with refractory angina due to advanced CAD accrue no symptom benefit after CSR implantation.88,89 The underlying reasons remain to be elucidated, but several potential explanations have been suggested, including the presence of well-developed alternative venous drainage of the left ventricle through the Thebesian system, inappropriate patient selection, inappropriate coronary sinus size, incomplete device endothelialisation, CAD progression and limited myocardial ischaemia at baseline. Further research is needed to understand the mechanisms responsible for non-response, which may help optimise selection of patients who are likely to benefit from CSR implantation.88,89
The Current Role of Reducer in Modern Interventional Practice
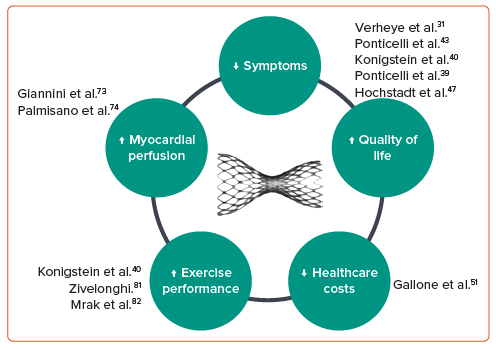
Current available data consistently support the clinical efficacy and procedural safety of CSR implantation (Figure 4). With emerging evidence explaining its mechanism of action and ability to address an unmet clinical need, it is clear there is a role for CSR implantation in the contemporary interventional management of patients with refractory angina secondary to advanced coronary artery disease. Its use in this well-defined population of patients is supported by guideline recommendations.49,50 Stronger guideline recommendations and expansion of CSR for new indications will require additional supportive data.49
Appropriate patient selection for CSR implantation will maximise the chances of treatment response. We suggest that a stratified approach to the selection of patients with refractory angina who have undergone detailed clinical review to confirm that symptoms are angina, assess angina burden, optimisation of medical therapy and investigations to identify the presence and underlying mechanisms of ischaemia is optimal for selection of patients most likely to benefit from CSR implantation. The presence of ischaemia should ideally be assessed using quantitative stress perfusion (Figure 5).
Patients should be reviewed in an appropriately constituted multidisciplinary Angina Heart Team.2,90 Many patients referred for CSR implantation will also have complex coronary anatomies, including previous bypass grafting and chronic total occlusions (CTO). CSR implantation has been shown to improve symptoms in a high proportion of patients with non-revascularised CTO.91 Angina Heart Team discussion should balance the risks of complex revascularisation against a low-risk CSR implantation when the therapeutic goal is primarily to achieve symptom and quality of life improvement.
Patient preference also needs to be carefully considered in the decision-making process. CSR procedures should be performed by appropriately trained operators in centres capable of delivering a comprehensive multidisciplinary care model designed to select appropriate patients and address the complex multifactorial care needs of patients with refractory angina.90
Knowledge Gaps and Current Research
When evaluating therapies, the importance of inclusion of sham controls, blinding and objective endpoint assessment has been demonstrated in recent pivotal trials, including COSIRA.92–97 This now establishes the contemporary standard of clinical evaluation by which emerging technologies need to be assessed before being strongly recommended in clinical guidelines and will therefore need to be the required standard for new studies evaluating the CSR.
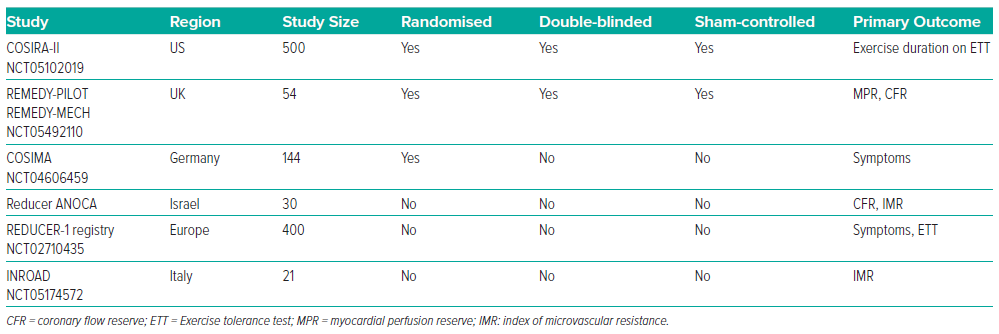
The CSR device was granted a CE mark in 2011 and has since been available for implantation in patients in the EU. In 2020, the CSR device was assessed by the Food and Drug Administration, which highlighted several areas that warranted further investigation, including further mechanistic data, the need for objective primary endpoints beyond CCS class and data from patients who represent the diversity of the US.98 Several on-going studies will address these knowledge gaps (Table 1).87,99,100
To confirm clinical efficacy, COSIRA-II (NCT05102019), a multicentre, randomised, double-blinded, sham-controlled trial is enrolling exclusively in North America, which will compare the effect of CSR on the primary outcome of change in total exercise duration on treadmill exercise test. Two imaging sub-studies (PET and CT) in a non-randomised arm will provide additional insights into safety and mechanism of action and allow recruitment of 270 patients additional to the 380 already planned.
With the observation that CSR implantation impacts the coronary microcirculation, the benefit of CSR implantation for patients with CMD has been suggested.85 Potential conditions include CMD in the context of ischaemia and non-obstructed coronary arteries, with concurrent CAD, or due to structural abnormalities such as hypertrophic cardiomyopathy.101 An unblinded randomised trial is recruiting in this space (COSIMA, NCT04606459).
Finally, CSR implantation is currently being investigated in a randomised, double-blinded, sham-controlled trial. The REMEDY-PILOT trial (NCT05492110) will test a primary efficacy outcome of change in myocardial perfusion assessed by quantitative perfusion CMR at 6 months between CSR and sham groups. A nested mechanistic sub-study will investigate change in invasive microvascular physiology and exercise physiology by CPET.
Conclusion
There is an increasing body of evidence consistently showing the clinical efficacy and safety of CSR implantation, improving angina severity and quality of life in patients with refractory angina who have exhausted conventional treatment options. This intervention is now established in current guidelines such that it may be considered for appropriately selected patients with refractory angina. On-going pivotal clinical outcome and mechanistic studies will address current knowledge gaps and provide new data for consideration by guideline committees. If favourable, we expect these data will lead to increasing adoption of CSR in contemporary clinical practice.