Tricuspid regurgitation (TR) is common, affecting 5% of the elderly population and, when its severity reaches moderate to severe, is an independent predictor of increased mortality.1–3 The pathophysiology of TR is mainly functional as it occurs in the context of pulmonary hypertension, left-sided heart disease and AF.4 These lead to deformation of the right ventricle (RV) and the right atrium (RA), which consequently causes dilation of the tricuspid valve (TV) apparatus.5–7
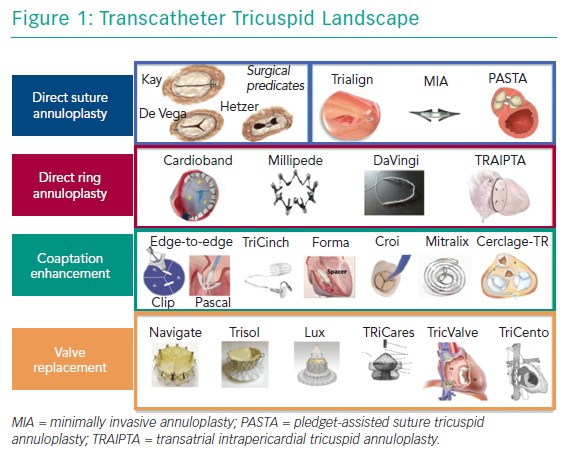
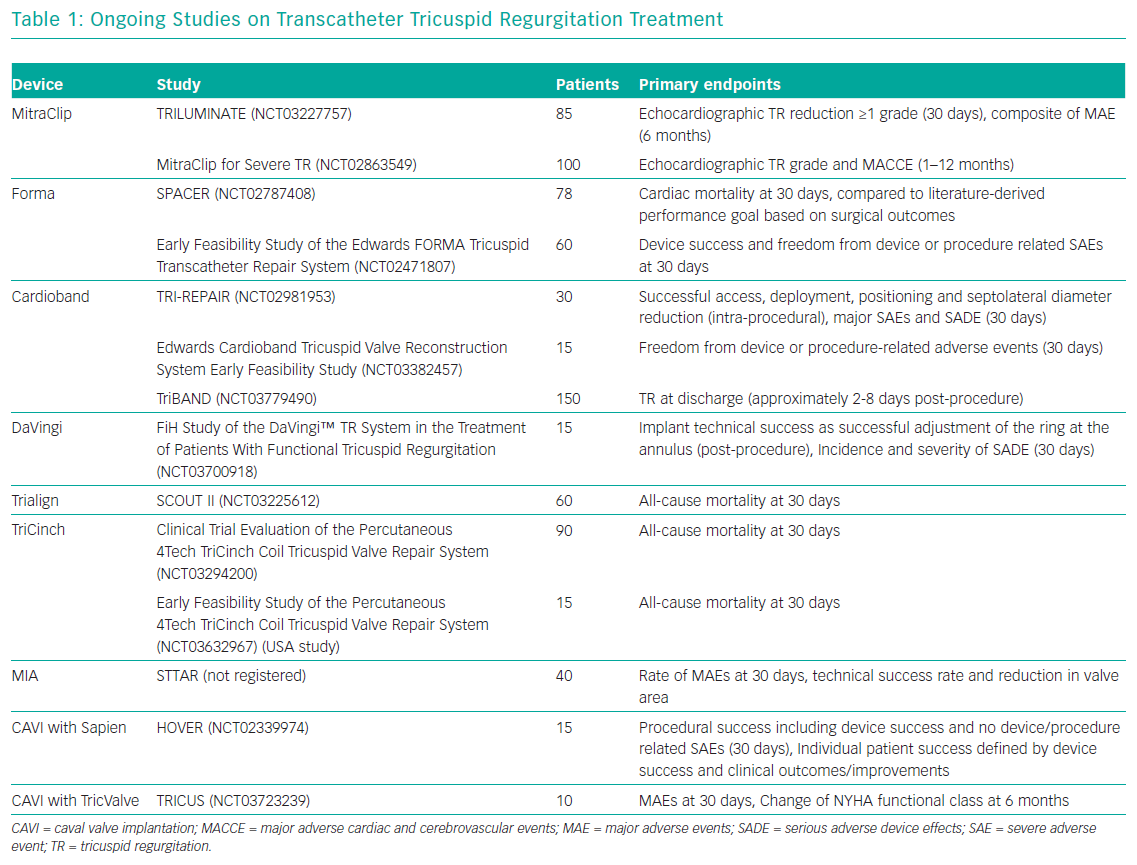
Currently, surgery is the only treatment option for patients who remain symptomatic on medical therapy. However, TV surgery is associated with an unacceptably high risk of operative mortality and poor outcomes.8,9 Therefore, TR remains noticeably undertreated and surgery in most cases is only performed as an additional procedure in concomitant left-sided interventions.10–12 Consequently, there is an unmet clinical need in patients with severe TR.13,14 A potential remedy in these patients may be new transcatheter options, which offer a less invasive alternative that could be used in high-surgical risk patients. At present, a wide range of devices are under development or under evaluation in first-in-human studies; of note, only one has been approved in Europe for the treatment of TR (Figure 1 and Table 1).15,16 Several concepts are targeting the tricuspid leaflets or the tricuspid annulus (TA) to repair the valve or replace it in an orthotopic or heterotopic position.
The aim of this article is to provide an overview of all devices and approaches for transcatheter TR treatment that are available or under development.
Evolving Transcatheter Options for Tricuspid Regurgitation Treatment
There are two main approaches with different subgroups that exist for percutaneous TR treatment – repair (i.e. leaflet approximation or annuloplasty) and replacement (in the orthotopic or heterotopic position; Figure 1).
Repair
Leaflet Approximation/Coaptation Devices
MitraClip in the Tricuspid Position
The MitraClip system (Abbott Vascular) is well established for the percutaneous treatment of degenerative and functional mitral regurgitation (MR) in patients at a high surgical risk.17–20 Extensive operator experiences as well as widespread availability have led to its use in the tricuspid position in more than 1,000 cases of severe TR. In the TriValve registry, which merged data on transcatheter tricuspid repair from 18 centres, the MitraClip was used in 66% of patients.21
Using one or more clips resulting in bicuspidisation of the valve by approximation of anterior and septal leaflets, or mimicking the clover technique by connecting the septal with the anterior and posterior leaflets, resulting in a triple orifice, are possible.22,23
The best results appear to occur by approximating the anterior and/ or posterior leaflet to the septal leaflet, which also potentially reduces annular dimensions. Clipping the antero-posterior leaflets is generally avoided in functional TR because it may distort the valve and worsen TR.
A multicentre European registry showed reduction of TR by at least one grade in 91% of patients, accompanied by a significant reduction in effective regurgitation orifice area, vena contracta (VC) width and regurgitant volume, as well as an improvement in New York Heart Association (NYHA) class and 6-minute walk distance.24 Of note, predominantly central and anteroseptal jets have been identified as predictors of procedural success.25 In many cases, procedures were successfully performed in combination with mitral valve repair.24,25
A modified version of the MitraClip device and its delivery system to address the specifics of TV anatomy is under investigation in the multicentre Evaluation of Treatment With Abbott Transcatheter Clip Repair System in Patients With Moderate or Greater Tricuspid Regurgitation (TRILUMINATE) study (NCT03227757) which has completed enrolment in Europe.26
Pascal
The repositionable and recapturable Pascal system (Edwards Lifesciences), initially designed for the treatment of MR, incorporates design features of the MitraClip. It has two paddle-shaped, independently closable, grasping arms (called clasps) as well as a central spacer that is intended to fill the regurgitant jet area.27
The first case of the Pascal system in the tricuspid position was reported in early 2018 and, recently, a case series of 12 patients was presented.28–30 In seven patients, two devices were implanted; four patients received one device and one procedure was unsuccessful because of imaging problems. TR reduction of ≥1 grade was achieved in 92% in the six patients, which persisted at 30-day follow-up. Twothirds of the devices were placed between septal and anterior leaflets, while one-third were placed between septal and posterior leaflets. One device detached while the patient was in hospital.30 Despite promising initial results, more data on the device’s durability and safety as well as an evaluation in a clinical trial are needed.
TriCinch
The TriCinch Coil System (4Tech Cardio), which is delivered through the femoral vein, is a second-generation update of the initial TriCinch device.31–33 While the first-generation device was anchored in the TA using a corkscrew-shaped anchor, this updated version is secured with an epicardial coil with two haemostasis seals. After the coil is implanted in the mid-anterior part of the TA, a nitinol stent, connected to the coil through a Dacron band, is placed in the inferior vena cava (IVC), to maintain tension applied to the TA. To enable safe deployment of the coil and ensure visibility in the epicardial space, a controlled pneumopericardium can be created using CO2.34 However, this device has limitations, including dehiscence and pericardial bleeding.
In the Percutaneous Treatment of Tricuspid Valve Regurgitation With the TriCinch System™ (PREVENT) trial (NCT02098200), 24 patients were treated with the first-generation device.35 Successful implantation was achieved in 18 cases with a significant (≥1 grade) reduction of TR in 94% of them. Two patients experienced haemopericardium after the procedure and, in four patients, late detachment of the TA anchor was observed. Follow-up at 6 months showed significantly improved 6-minute walk distance and quality of life (QoL), with 75% of the patients being in NYHA class I or II. Firstin- human cases of the second-generation device at 30-day follow-up have shown maintained significant TR reduction from severe to mild as well as improvements in NYHA class (from III to I) and QoL.34,36
Two trials are enrolling patients to generate further safety and performance data (NCT03294200 and NCT03632967).
Forma Spacer
The Forma Repair System (Edwards Lifesciences), which is placed in the regurgitant orifice and creates a new surface for leaflet coaptation, consists of a foam-filled spacer that is inserted via the subclavian or the axillary vein and, after successful deployment, is anchored in the RV apex.37
The first-in-human experience dates back to 2015.38 Procedural success was achieved in 16 (89%) of 18 treated patients.39 In one of the two unsuccessful procedures, right ventricular perforation required conversion to open heart surgery. Sustained reduction to moderate-to-severe TR was present in only six (46%) of 13 assessed patients after 1 year. However, in 79% of patients, significant reduction in NYHA class, increase in 6-minute walk distance and improvement in heart failure symptoms were observed at 1 year.39 Similar results were obtained after 1 year in the US Forma early feasibility study (NCT02471807), which enrolled 29 patients.40 Long-term outcomes at 24–36 months of the first-in-human cohort showed significant improvement of functional status and significant reduction of VC width from ‘massive’ to ‘severe’ values.41,42 Additional data is expected from the Repair of Tricuspid Valve Regurgitation Using the Edwards TricuSPid TrAnsCatheter REpaiR System (SPACER) trial (NCT02787408).
The initial findings have led to several modifications to the secondgeneration device: larger spacers are available to address ‘torrential’ forms of TR; and anchoring is improved by a new sheath as well as by a radiopaque apposition indicator.40,43 This new iteration of the Forma device will have to prove its safety and efficacy in a future study.
Cerclage TR-block
The Cerclage-TR block (Tau-PNU Medical) is an experimental device being evaluated in porcine models.44 It is based on the platform of the Mitral Loop Cerclage Annuloplasty device (Tau-PNU Medical), which has been shown to be able to significantly reduce MR in four of five patients studied in a first-in-human study.45,46 This device is anchored in the subclavian vein and creates a loop structure surrounding 360° of the mitral annulus. One arm of the device enters the coronary sinus, then the great cardiac vein and, finally, a septal vein. This vein is perforated, the septum is traversed and the device is snared in the RV outflow tract. Here, the first device arm is connected with the second device arm that originates from the RA, crosses the TV plane and is positioned underneath the septal tricuspid leaflet. This cerclage then can be cinched to reduce MR. The Cerclage-TR block adds a crescentshaped ‘T-leaflet’ to the device arm crossing the TV plane.
This T-leaflet serves as an extension of the septal leaflet and improves coaptation of the native tricuspid leaflets. In preclinical (n=5) and isolated heart (n=5) models of functional and degenerative regurgitation, TR reduction by at least 1 grade was observed.44 The elegance of this procedure is the combined treatment of TR and MR with a single device. However, clinical first-in-human data is needed to further explore the potential and shortcomings of this approach.
Mistral
The spiral-shaped Mistral device (Mitralix) targets the sub-valvular chordae tendinae of diverged tricuspid leaflets and is delivered via an 8.5 Fr delivery system. Its spiral is rotated in the RV to grasp the chordae of two adjacent leaflets that are pulled together to improve coaptation.
While it was originally intended for the mitral position, two first-inhuman cases addressing TR have now been presented.47–49 In both cases, the device was implanted at the antero-septal commissure, resulting in a TR reduction from ‘severe-to-massive’ to ‘mild-tomoderate’ in the first case and from ‘severe-to-massive’ to ‘moderateto- severe’ in the second case.49 Further clinical evaluation to establish the safety and efficacy of the Mistral device are required as well as an evaluation of whether multiple devices may be necessary.
CroíValve
The preclinical CroíValve system (CroíValve) aims to assist coaptation of dilated tricuspid leaflets.50 It is anchored in the superior vena cava (SVC) and placed between the native leaflets to reduce the size of the regurgitation orifice and provide a surface for coaptation. In addition to being a pure spacer, CroíValve has an inner part that consists of a tri-leaflet valve apparatus to support diastolic forward flow through the TV and thus potentially reduce the risk of device thrombosis.
In-vivo and ex-vivo preclinical testing in a porcine model showed significant TR reduction and 30-day follow-up showed long-term stability of the anchors as well as atraumatic coaptation with the native leaflets. In advance of first-in-human cases, longer-term animal studies are planned.50
Annuloplasty Using Rings
Cardioband
The Cardioband Tricuspid Repair System (Edwards Lifesciences) is the first transcatheter device that received CE mark approval for TR treatment, following promising results in the mitral position for the treatment of MR.51,52 It consists of a Dacron, surgical-like band that is implanted at the atrial side of the tricuspid valve by advancing up to 17 screws through the band into the tissue of the TA. After implantation at the anterior and posterior parts of the TA using fluoroscopy and transoesophageal echocardiography guidance, the device is cinched to reduce anteroposterior and septolateral diameters.
Six-month results of the TRI-REPAIR trial were recently reported.53 Technical success (including access, deployment and positioning) was achieved in 100% of the 30 enrolled patients. At 30-day follow-up, one device-related death was observed. Significant reductions of TA diameter, effective regurgitation orifice area and VC were sustained at 6-month follow-up. Furthermore, there were significant increases in 6-minute walk distance and Kansas City Cardiomyopathy Questionnaire scores, and a reduction in NYHA class at 6-month follow-up.53
Similar to the mitral position, staged procedures combining a Cardioband implantation with subsequent MitraClip implantation in the tricuspid position have been shown to be possible, resulting in significant reduction of TR severity.54–56
Besides the European TrIcuspid Regurgitation RePAIr With CaRdioband Transcatheter System (TRI-REPAIR) trial (NCT02981953) and the Transcatheter Repair of Tricuspid Regurgitation With Edwards Cardioband TR System Post Market Study (TriBAND) post-market study (NCT03779490), a US-based study evaluating early feasibility is under way (NCT03382457).
Iris
The Iris Transcatheter Annuloplasty Ring (Millipede) consists of a semirigid complete ring. Anchors are screwed into the TA and a zigzagshaped ring attached to the screws. Collars attached to each angle of the zigzag ring can be adjusted to reduce the diameter of the frame and to approximate neighboring screws, thereby cinching the TA.
To date, the Iris ring has been implanted surgically in two patients in a combined procedure to treat TR and MR. Significant reduction in TR to trace degrees was noted, with an average TA diameter reduction of 36% that was stable at 6-month and 12-month follow-up.57,58
A dedicated catheter for the tricuspid space is under development. These promising initial results will have to be proven in larger patient cohorts.
Transatrial Intrapericardial Tricuspid Annuloplasty
Transatrial intrapericardial tricuspid annuloplasty (TRAIPTA; National Institutes of Health and Cook Medical) is a fully retrievable transcatheter system for indirect tricuspid annuloplasty in the pericardial space. Access to the pericardium is gained through a puncture of the right atrial appendage. Then, the actual implant, consisting of a hollow tube of braided nitinol wire, which allows for a longitudinal shortening of more than 50%, is placed in the atrioventricular groove. After the device has been tightened, the delivery system is retrieved, and the atrial appendage puncture is closed using nitinol or bioresorbable occluders.
In nine naive pigs, significant reductions of anteroposterior and septolateral diameters, TA area and perimeter were achieved, while leaflet coaptation length was significantly increased. In an additional four pigs with functional TR, the severity of regurgitation was reduced.59 There are plans for a first-in-human evaluation.60
DaVingi
The DaVingi neo-annulus (Cardiac Implants) is a two-stage concept that was initially presented in 2015 for use in the tricuspid space.61 In the first stage, a flexible ring with an internal adjustment chord is delivered to the atrial side of the TA using a 22 Fr catheter featuring a distal balloon to support stabilisation of the device; this is attached to the annulus by simultaneously firing all the anchors. The resulting chronic healing response creates a tissue bond to secure the implant. The adjustment chord is fixed at the jugular venous access site. In the second stage, the final cinching of the neo-annulus is performed using a dedicated 16 Fr adjustment device. A first-in-human study is ongoing; the first five patients are being enrolled (NCT03700918) and an early feasibility study has been filed with the Food and Drug Administration (FDA).62
Annuloplasty Using Other Approaches
Trialign
The Trialign device (Mitralign) uses transcatheter suture annuloplasty to mimic surgical Kay bicuspidisation. Through jugular vein access, an insulated radiofrequency wire is advanced into the RV to then retrogradely cross the TA tissue. In this way, two pledgets are placed at the posteroseptal as well as the anteroposterior commissures, which are then cinched to obliterate the posterior tricuspid leaflet, creating a bicuspid valve and thus reducing TR.63,64
In the Early Feasibility of the Mitralign Percutaneous Tricuspid Valve Annuloplasty System (PTVAS) Also Known as TriAlign™ (SCOUT) trial (NCT02574650), implantation success was achieved in all 15 patients. However, one patient required right coronary artery stenting because of extrinsic compression. At 30-day follow-up, the technical success rate was 80%; three patients developed single-pledget TA detachment, but they did not require reintervention.65 In the remaining patients, significant reductions of TA areas and effective regurgitation orifice area were observed while left ventricular stroke volume improved. In addition, NYHA class, 6-minute walk distance and QoL were significantly improved. After 12 months, one nondevice or procedure-related death was recorded and one patient received elective reintervention. Improvements in NYHA class and QoL were sustained at follow-up.66 It has been postulated that the problem of pledget dehiscence may be resolved by optimising pledget distance during implantation.67
The small footprint of the device, which leaves a significant amount of native anatomy undisturbed for subsequent procedures, is a notable advantage.
The Safety and Performance of the Trialign Percutaneous Tricuspid Valve Annuloplasty System (PTVAS) (SCOUT-II) trial (NCT03225612), which will include 60 patients from up to 15 sites to further assess safety and performance of the Trialign device, has started enrolment.
Minimally Invasive Annuloplasty with PolyCor Anchors
Minimally invasive annuloplasty (MIA™) technology (Micro Interventional Devices) consists of low-mass, polymeric, self-tensioning PolyCor anchors and the thermoplastic MyoLast polymer for tensioning of the anchors.
In the completed first arm of the Study of Transcatheter Tricuspid Annular Repair (STTAR) trial assessing surgical deployment of the twobarbed PolyCor anchors in three patients, feasibility was proven, as implantation was successful in this small cohort without any adverse events or anchor detachment. An average 43% reduction in TA area was achieved with a reduction of TR from severe and moderate to mild/trace at 6-month follow-up.68
The percutaneous version of the device, deployed using a dedicated 12 Fr delivery catheter, is aimed at creating a bicuspidisation of the TV by implanting multiple PolyChor anchors in the region between the posteroseptal and anteroposterior commissures that are tensioned after deployment, resulting in an obliteration of the posterior leaflet.
Enrolment of 40 patients in the percutaneous arm of the STARR trial has started and the first four procedures were recently performed.69 In these four patients, no adverse events have been recorded so far and significant TR reductions to mild/moderate with TA area reductions of 48% were reported. Further results from this study will show if the unique PolyChor anchoring design has any advantage over other anchoring approaches.
Pledget-Assisted Suture Tricuspid Annuloplasty (PASTA)
The transcatheter pledget-assisted suture tricuspid annuloplasty (PASTA) technique mimics the surgical Hetzer double-orifice suture using marketed devices. Sutures and pledgets are placed at midanterior and posterior-septal parts of the TA in a ‘double-bite’ manner to enhance pull-through force. The suture limbs are then tightened and secured using a Cor-Knot device (LSI Solutions).70
In 22 pigs, the concept was proven and distinct reductions in TA dimension and TR were achieved.71 Recently, first-in-human compassionate use cases were performed but suture dehiscence remains an important limitation.72 This challenging technique might present a future possibility in selected patients who lack other options.
Orthotopic Transcatheter Tricuspid Valve Replacement
NaviGate
The Gate self-expanding bioprosthesis (NaviGate Cardiac Structures) for orthotopic tricuspid valve replacement consists of a tapered nitinol stent with atrial winglets and ventricular graspers for anchoring the TA and leaflets and carries three xenogeneic pericardial leaflets. The device currently is the only TV replacement system with firstin- human experience and is available in four sizes intended for TA diameters ranging from 36 mm to 52 mm, with oversizing of around 2–5% performed. A 42 Fr introducer sheath is used to deliver the valve through a transjugular or surgical transatrial approach (minimally invasive right thoracotomy). The delivery system features two degrees of motion at the tip and allows for a 90° angulation.73 An extensive description of relevant imaging and procedural steps was recently published.74
First-in-human cases were performed in 2016 and 2017 and, cumulatively to date, 32 patients have received a Gate valve on compassionate grounds.75,76 After baseline severe or torrential TR in all patients, postoperative TR was mild or less in 96% and moderate in the remaining 4%. Postoperative NYHA class improved to I or II in 91% of the patients and 30-day mortality was 12.5%. A single-site experience in five patients recently reported RV remodelling, increased cardiac output and sustained NYHA class improvement in four patients who survived until 30-day follow-up; it also found that poor baseline RV function was associated with worse outcomes.74 While the right coronary artery is not compromised by the implantation, oversizing of the Gate valve may cause damage to the conduction system or the ventricular septum. Therefore, nominal sizing may be used, which, considering the danger of paravalvular regurgitation, is probably better tolerated in the low-pressure system of the right heart.
Device iteration and a smaller dedicated delivery system with a greater degree of flexion for transjugular or transfemoral approach may improve outcomes. Further data including long-term follow-up are needed to identify the optimal patient population for this device.
TriSol
The TriSol valve (TriSol Medical) is mounted on a self-expanding nitinol stent featuring a ventricular skirt of porcine pericardium and an atrial polyester skirt. The conical stent shape conforms to native anatomy, and anchoring through axial instead of radial forces protects the conduction system. The retrievable and repositionable valve is delivered through the jugular vein using a 30 Fr delivery system. The unique valve concept consists of a single bovine pericardial structure with a single dome-shaped leaflet that is attached in two opposite central commissures to create a bileaflet valve.
During diastole, the two leaflets move to the centre of the valve and open two lateral orifices for RV inflow. During systole, the leaflets coapt with the circumference of the cone-shaped stent forming a dome shaped structure that enables a larger RV closing volume.77 This is expected to offer RV pressure relief and preserve RV function, which are needed as the sudden increase in afterload after TV replacement may lead to RV failure resulting in poor long-term outcomes.78
In 12 acute swine models, the valve was successfully deployed and, in four chronic swine models, up to 5 months’ follow-up showed stable outcomes in healthy animals without signs of heart failure.79 A first-inhuman implantation after further preclinical testing is planned for 2020.
LUX-Valve
The LUX-Valve (Jenscare Biotechnology) is a self-expanding bovine pericardial tissue valve on a nitinol stent covered by a layer of polyethylene terephthalate. After transatrial insertion via a minimally invasive right thoracotomy, it is secured in the upper part of the RV using a D-shaped special anchoring mechanism that attaches it to the interventricular septum. A self-adaptive skirt to avoid paravalvular regurgitation encircles the valve.
In an experimental animal study, 10 goats showed promising results, with nine goats surviving for more than 6 months.80 Long-term effects and possible first-in-human use need further evaluation.
TRiCares
The TRiCares valve (TRiCares SAS, Paris, France) is a self-expanding bovine pericardial valve mounted on a nitinol stent frame with atrial and ventricular widening to enable secure anchoring in the TA. Firstin- human use of the TRiCares valve is being planned.
Heterotopic Tricuspid Valve Replacement
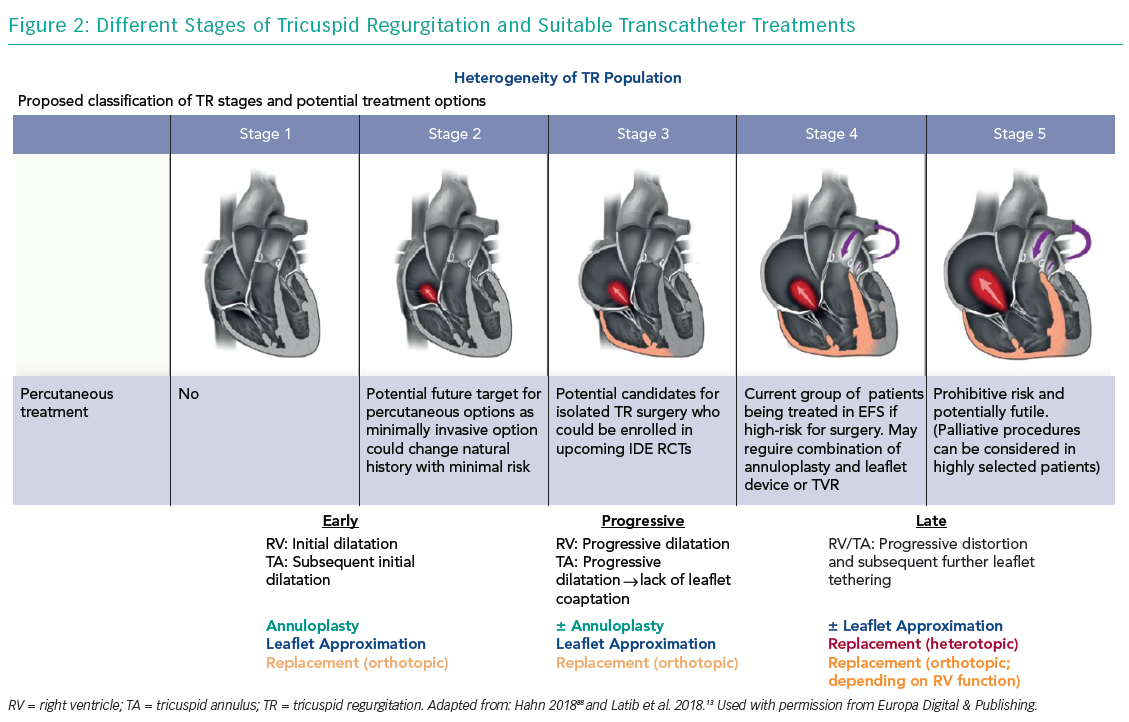
Heterotopic caval valve implantation (CAVI) does not directly address TR but is intended to lower caval backflow of TR by implanting a valve in the IVC and SVC, thus reducing overload of the venous system. However, as this does not treat the underlying pathology of TR and results in a chronic overload of the RA as well as consequently increased RV afterload, CAVI is reserved for advanced stages of TR (Figure 2).
Sapien Off-label Use
In heterotopic off-label use, 29 mm Sapien valves (Edwards Lifesciensces; approved for transcatheter aortic valve replacement) were implanted in the IVC in two first-in-human cases, and in the IVC and SVC in a third first-in-human case.81 Results of the randomised Treatment of Severe Secondary TRIcuspid Regurgitation in Patients With Advance Heart Failure With CAval Vein Implantation of the Edwards Sapien XT VALve (TRICAVAL) trial (NCT02387697), which compared Sapien valve implantation in the IVC to optimal medical treatment, have been recently reported.82 The trial was prematurely ended after 28 patients had been enrolled, as no differences were found in eight patients with CAVI at 3-month follow-up in oxygen uptake, NYHA class or QoL compared to medical treatment.
Results of the Heterotopic Implantation Of the Edwards-Sapien Transcatheter Aortic Valve in the Inferior VEna Cava for the Treatment of Severe Tricuspid Regurgitation (HOVER) trial (NCT02339974), which is assessing the short-term safety (<30 days) and efficacy (6 months) of IVC CAVI with Sapien valves in 15 patients, are awaited.
TricValve
Limitations of off-label use of other valves and the complexity of caval anatomy reiterate the need for dedicated CAVI valves. The TricValve (P&F Products & Features) device consists of two pericardial tissue self-expandable valves on a nitinol stent frame, one specifically for the IVC and one specifically for the SVC.83 The valves have little radial force and do not require pre-stenting of the vena cava.
First-in-human experience showed immediate abolition of caval backflow and clinical improvement at 12-month follow-up in a patient with SVC and IVC valves in areas such as NYHA class, hepatic synthetic function and 6-minute walk distance.84,85 The Safety and Efficacy of the TricValve® Device (TRICUS) study (NCT03723239), assessing safety and efficacy of the TricValve, recently started to enrol around 10 patients.
Tricento
The Tricento (NVT) CAVI device consists of a bicavally anchored nitinol stent deployed top down from the SVC to the IVC. A lateral bicuspid porcine pericardium valve permits inflow into the RA. The device is inserted transfemorally through a 24 Fr delivery system. It needs to be custom made for each patient so has a high cost.
After successful evaluation in seven bovine models, a first-in-human case was recently reported.86,87 A 74-year-old woman received a Tricento valve without procedural complications. The caval vein regurgitant volume was reduced from 50 ml to 24 ml and renal function as well as functional capacity improved. At 3-month follow-up, the prosthesis was in place without transvalvular regurgitation; however, after 4 months, the patient died from terminal kidney failure.
Outlook
Several devices that adopt multiple approaches to treat TR are under development or investigation and it is too early to predict which device or approach will succeed. Several repair devices are leading the field as the first clinical data including mid-term follow-up are available, but replacement devices are catching up.
While repair devices, especially in experienced hands, have specific advantages (e.g. the ability to target individual anatomy, preservation of native valve structure and greater operator experience), replacement devices theoretically promise significant advantages (e.g. a combined platform for primary and secondary TR, reproducibility, a standardised procedure, an easier learning curve and complete elimination of TR) and different approaches might be applicable for different TR stages (Figure 2). However, the risk of afterload mismatch, valve thrombosis and the long-term impact on RV function are potential limitations of replacement.
As first-generation devices are assessed in clinical trials, identification of correct and clinically relevant endpoints is crucial. A new TR grading addressing the different stages of severe TR might be necessary to measure TR improvement after the procedure and, furthermore, a detailed QoL assessment is fundamental to elucidating procedural benefits.42 Principally, to enable sufficient reimbursement and widespread use, devices will have to prove themselves in comparison to optimal medical treatment or maybe even surgical intervention.
In summary, as the vast unmet clinical need is obvious, development and investigation of devices to treat TR will continue. No device or approach is clearly leading the field, with some repair devices as first among equals because of the availability of promising initial clinical data.