Left ventricular (LV) unloading of the heart after acute MI and prior to reperfusion reduces infarct size by decreasing LV wall stress and improving coronary perfusion, thereby reducing the magnitude of ischaemia–reperfusion injury.1 There is an unmet clinical need to understand the effects of reloading after unloading occurs.
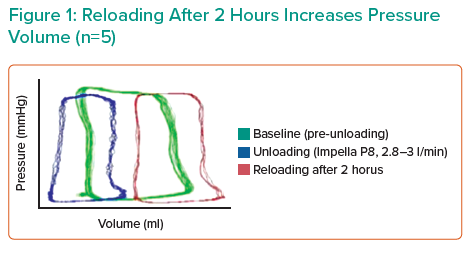
Dr Mazurek’s hypothesis is that acute reloading is detrimental to the heart. Haemodynamic measurements and tissue samples were collected from six Yorkshire pigs that underwent unloading with Impella CP for 2 hours, followed by device removal and reloading for 2 hours. Preliminary results indicated a 45% increase in end-diastolic wall stress (EDWS) after 5 minutes of reloading and increased pressure–volume area with decreased systolic function after 2 hours. A significant increase in apoptotic markers, including DNA fragmentation, Puma and caspase-3 was also observed in the reloading-alone group. These data indicate that acute reloading has a negative impact on the normal heart.
Dr Mazurek’s hypothesis is that gradual reloading can mitigate deleterious apoptotic and EDWS effects induced by rapid reloading of the ventricle.
Future studies will include investigating the effects of acute reloading and gradual reloading in animal models of acute MI (AMI). Measurements of haemodynamics, apoptosis and infarct size 1 week after AMI will be collected from six Yorkshire pigs with coronary artery occlusion. These animals underwent unloading with Impella CP for 2 hours, followed by either consecutive acute reloading and reperfusion or consecutive gradual reloading using Impella-mediated pressure adjustments and reperfusion. Acute study endpoints will assess apoptosis and no-reflow area, whereas chronic study endpoints will include infarct size, LV function and ventricular remodelling.
Dr Mazurek’s abstract presentation was awarded an Acute Cardiac Unloading and REcovery Research Grant.