Cardiovascular diseases represent the leading cause of mortality in pregnancy.1,2 They are classified as direct (acute onset disease) or indirect (worsening of pre-existing cardiac disease). This overview is focused on acute coronary syndrome (ACS) in pregnancy and the post-partum period. ACS in this setting is a rare condition, but in the last two decades its incidence has grown.3 This phenomenon may be partially due to a higher diagnostic accuracy secondary to the advent of troponin monitoring and increasing availability of catheter laboratories. On the other hand, pregnancy is a condition that may worsen the presence of atherosclerotic disease (AD) or impair coronary blood flow, yielding spasm, thrombus formation or spontaneous dissection. Finally, the increased pregnancies in women over 35 years who already have cardiac risk factors is a growing phenomenon.4
Generally ACS occurs during the third trimester of gestation and extends until the second month post-partum. Although peak incidence have been reported between the last month of gestation and the first two weeks post-partum.5 Clinical presentation vary from unstable angina (UA), non-ST elevation myocardial infarction (NSTEMI) to ST elevation myocardial infarction (STEMI), which may be complicated by cardiogenic shock and death. The diagnosis is often difficult either because of its abrupt onset in non-suspected cohort or because of the fact that gestational status may mimic a variety of symptoms.
Epidemiology
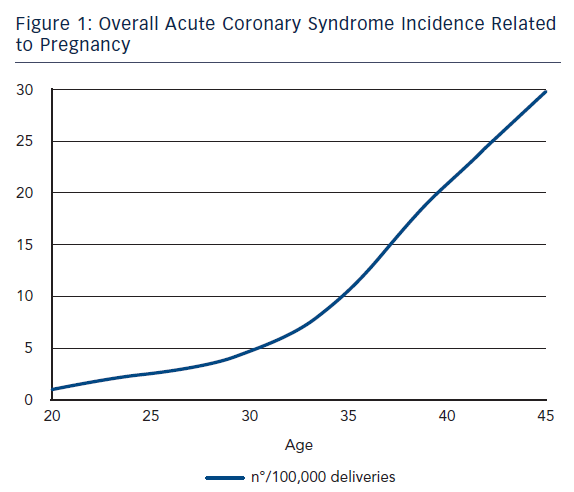
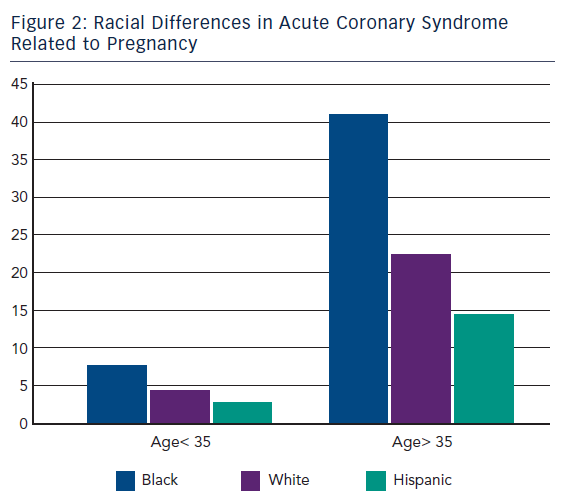
ACS during pregnancy is considered rare, representing only a small part of the adverse events. Its incidence ranges from 3 to 10 per 100,000 deliveries6 with high rates of maternal and foetal mortality.7 A recent US population-based study revealed that about two-thirds of ACS occurred during pregnancy whereas one-third occurred during the post-partum period.3 This study showed that between the years of 2000 and 2002 only 45 % of women with ACS underwent coronary angiography and 37 % received percutaneous or surgical treatment. Deepening for racial differences; black woman had the highest risk for ACS (11.4 cases every 100,000 deliveries) followed by white woman (7.6/100,000) and then Hispanic woman (4.2/100,000).3 Furthermore, age was shown to be a strong risk factor for ACS since its incidence increased 5–8 times in women aged >35 years bereft of racial differences.3,8
Brief Overview of Cardiovascular Physiology During Pregnancy
Pregnancy is a new physiological state. A previously published review extensively focused on this topic.12
- volume changes;
- sympathetic activation;
- pro-coagulative state; and
- changes in connective tissue.
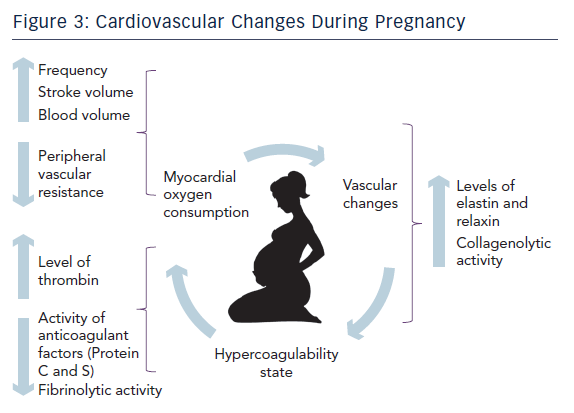
Firstly, a mother’s body needs to accommodate and allow sufficient nutrients to the foetus. For this reason there is an increased erythropoiesis and plasma volume. Total body liquids, including plasma, increase by almost 7–8 litres. These changes result in about 50 % cardiac output increase. 13
Secondly, during delivery heart work increases by 70–80 % due to either sympathetic stimulation secondary to pain and anxiety or volume overload caused by 300–500 millilitres of blood rejected after each uterine contraction. At the same time vascular peripheral resistance reduces and heart rate increases.2
On the other hand, there is an increased level of clotting cascade factors resulting in a higher generation of thrombin14 while anticoagulant factors such as protein S and C reduce their activity. Moreover there is a decreased production of tissue plasminogen activator.15 Finally placenta contributes to produce more plasminogen activator inhibitor reducing fibrinolytic capacity.16,17 These physiological changes in haemostatic cascade are helpful in view of the natural need to reduce haemorrhagic phenomenon during the last trimester, delivery and post-partum period.
The last step is designed to accommodate and facilitate foetus growth and delivery. The musculoskeletal system of the pelvis enhances its laxity by the influence of sexual hormones. They stimulate the release of relaxin and elastin hormones, which determine a remodelling of collagen fibres through the activation of the collagenolytic system. The latest similarly affects the fibrous tissue of the vascular system as well. This mechanism has been advocated as one of the most plausible hypothesis to explain SCAD.
Aetiology
ACS may be due to different causes.
Atherosclerotic Disease
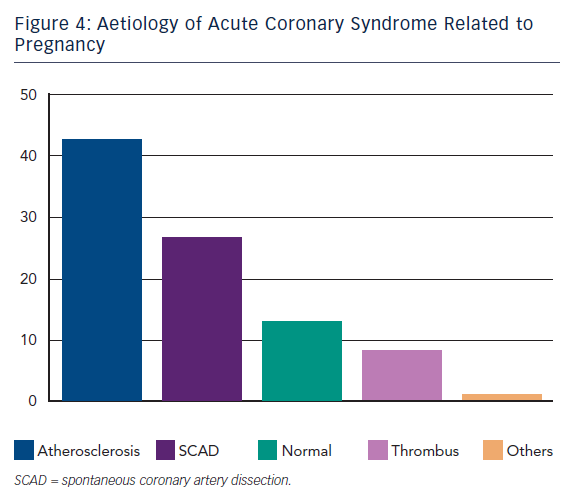
AD is the most frequent aetiology of ACS in the general population. Likewise it is shown to be prevalent even in pregnancy ACS in about 40 % of cases.9 Clinical presentation, risk factors, diagnosis and treatment are comparable to conventional ACS, widely described in the literature.
Spontaneous Coronary Artery Dissection
Epidemiology
SCAD is the second cause of ACS during pregnancy. It represents a challenge due to extremely heterogeneous clinical presentations and the difficulties to perform early diagnosis in patients usually with low cardiovascular risk factors. Initially reports consisted in only autopsy findings. The first case of sudden death in a young woman was described in 1931.18 Although mortality is still high the percutaneous intervention era has consistently reduced it from 48 % in the earliest reports19,20 to less than 20 % in the most recent reports.21–24 Interestingly, SCAD was not associated to hypertension but to elevated shear stress conditions such as physical effort,25–27 emotions and drug consumption28,29 (i.e. cocaine and methamphetamine). In other conditions it was associated to connective disorders,30 vasculitis31 or autoimmune diseases.32,33
Pathogenesis
The complete pathogenesis of SCAD is not yet fully understood. There is no clear correlation with the traditional cardiovascular risk factors. Nevertheless older pregnants (>35 years) and multiparous women seem to be at increased risk.34 Estro-progestinic hormones contribute to a prothrombotic status and laxity of collagen fibres of media arterial layer. This status under conditions of high shear stress, such as delivery, may facilitate SCAD. Autopsy findings of SCAD showed a dissection intersecting the deeper media layer toward the adventitia.35 Moreover in almost 50 % of the cases an eosinophilic inflammatory infiltrate in the site of dissection was detected.36,37 This evidence suggests a role for collagenolytic enzymes released during eosinophil degranulation into the media layers altering the reticular fibres.38–40 On the other hand, a reduced production of collagen seems to characterise the pregnancy.41 Nevertheless these authors do not exclude that these findings might also be a consequence of dissection. SCAD might be occupied by liquid or organised blood due to vasa vasorum rupture causing haematoma. Thus a dissection and a false lumen are created. At this point the evolution is unpredictable and highly variable.21 Dissection might extend distal or proximal, linear or circumferential. If the haematoma grows without breaking the intima, it will compress the lumen. Otherwise the haematoma might open into the true lumen. In all cases the consequences can cause highly variable aspects ranging from the simple reduction of blood flow to the complete occlusion of the vessel.42 The occlusion may be facilitated since in the absence of AD coronary arteries they do not have the same wall stiffness thus more likely a dissection can evolve and cause a protruding flow limiting haematoma. Summarising previous reviews related to the hypothesis generating mechanism of SCAD, the authors support a role for all the above mentioned factors working together in generating SCAD.
Clinical Presentation
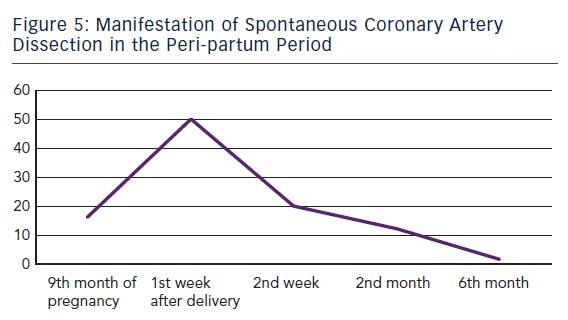
ACS caused by SCAD is indistinguishable from others. Sometimes even angiography does not clearly show the dissection, especially when it causes a total occlusion of the vessel. In this case intravascular ultrasound (IVUS), if carefully employed, can evidence mechanism of occlusion. In the largest meta-analysis to date, out of 440 patients with unselected SCAD about 80 % occurred in women – among them 80 were observed during pregnancy. In the later subgroup SCAD occurred mainly in the post-partum period (83.8 %) with 50.0 % and 70.0 % of them during the first and second post-partum week, respectively.11 Only 13 patients (16.2 %) developed SCAD before delivery.
Normal Coronary Arteries
About 13 % of ACS in this setting presented NCA at angiography.9 These findings may be partially explained by the presence of occlusive transient coronary spasm due to increased renin release and angiotensin production during uterine hypoperfusion in the supine position44 or secondary to vasoconstrictive agents used during pregnancies such as ergonovine.45 Furthermore thrombus creation and solution with distal embolisation/fragmentation cannot be ruled out in some cases considered as NCA.
Isolated Thrombus
Isolated thrombus without evidence of AD, SCAD or spasm was reported in about 8 % of cases.10 Aetiological explanation seems to be basically grounded on the role of the hypercoagulable state (HS). As mentioned above, a pivotal feature of pregnancy is an acquired HS dominant during the third trimester and the post-partum period. This condition might worsen a pre-existing inherited thrombophilia such as antithrombin III, protein C, protein S deficiency and factor V Leiden mutation, or an acquired one such as antibody anti-phospholipids syndrome or hyperhomocysteinemia.46,47 Nevertheless, pregnancy-related HS is temporary and generally recovers after puerperium. On this evidence it is reasonable to attribute a role for HS in pregnancy ACS even in the presence of AD or SCAD. This acquired state is considered an independent risk factor for ACS.3,5
Diagnosis
Diagnosis is often challenging as attention is mainly focused on pregnant women and children and not on young individuals with a low cardiovascular risk. Generally, presentation is abrupt without warning signs. Symptoms suggestive of ACS should always be investigated even if found in young healthy patients. An immediate emergency room evaluation should be performed when necessary. Attention should be oriented to exclude cardiac aetiology given the still high mortality. Electrocardiogram (ECG) is adequate to diagnose STEMI. In this case urgent coronary angiography, as in general ACS guidelines, is mandatory. In the setting of SCAD particular attention should be paid to non-diagnostic or atypical ECG during chest pain, since SCAD is very insidious and fluctuating from phases with no flow limiting dissection to variable degrees of obstruction to complete occlusion. This mechanism of disease could explain the variable features of ECG over time. Laboratory findings such as seriated troponin, creatine kinase (CK), creatine kinase muscle and brain subunits (CK-MB), fibrin degradation product (FDP) and brain natriuretic peptide (BNP) measurements are helpful to make a differential diagnosis or confirm ACS. Echocardiogram may give further information revealing regional wall motion abnormalities or ventricular dysfunction. If high-risk non-ST elevation ACS is confirmed, coronary angiography is mandatory to establish pattern of disease (SCAD, thrombosis, AD or spasm), its localisation and extension. Otherwise in case of low risk UA a watchful and waiting strategy is reasonable, maintaining a low indication threshold for coronary angiography. There is a different procedural risk in pregnant or post-partum patients with regard to radiologic exposure of the foetus,48 haemorrhagic risk and abortion hazard. Radial access and screen for uterus are strongly suggested in pregnants in order to reduce foetus x-ray exposure.
In the last decade the increasing use of coronary angiography has allowed us to better understand and diagnose ACS. Furthermore the adequate use of IVUS examination helped to better characterise the pattern of disease. However, based on the pathophysiology of SCAD, we suggest a careful implementation of IVUS prior to stent implantation in this subgroup of patients since no safety data exist and further spasm or dissection might occur.
Treatment
Current guidelines do not report any clear indication on the treatment of ACS in pregnancy.49–52 On the other hand, a few reports suggest a management according to general ACS guidelines.53,54 As a matter of fact no general consensus has yet been established. Based on the above mentioned pathology and clinical features we strongly believe that treatment in this setting should be well-tailored on a patient-to-patient basis. In dealing with ACS we have to keep in consideration two major modalities: the interventional/surgical aspect and the pharmacological aspect. Both may have restrictions in different periods of pregnancy. To facilitate management we believe that patients should be classified in pre- and post-partum groups.
In the post-partum ACS group treatment according to the general ACS guidelines49–52 is recommended since only the mother’s health is at risk and the only jeopardised function with regard to the newborn is lactation. Fortunately, the latter can be implemented by an artificial one. Thus, the physician is relatively facilitated either in terms of treatment intensity (i.e. use of double antiplatelet therapy or glycoprotein IIB/IIIA inhibitors) or in the choice of the type and number of stents required, such as drug eluting stents (DES), and finally surgery. On the contrary, the pre-partum ACS group is hampered by the presence of the foetus. These patients are at risk for spontaneous abortion, bleeding and teratogenic effects.9,55 Management should keep several factors in consideration.
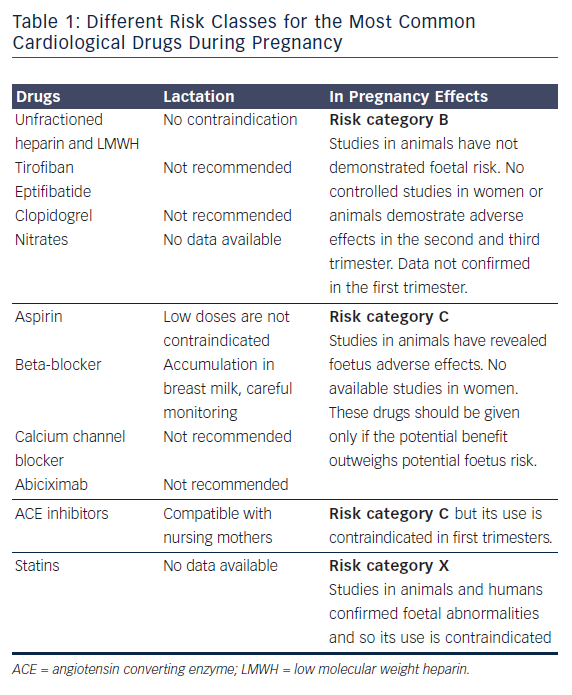
Regarding pharmacological treatment, there is limited information about most of drugs when used in pregnancy. Drugs have been divided in classes of risk ranging from A (potential foetal damage is remote) to D (administer only in life-threatening situation in absence of any other choice) and X (risk clearly outweighs the benefit).9
Actually, thrombolysis in pregnant is a relative contraindication for the high foetal and maternal haemorrhagic risk.59 Several cases in literature report ischaemic stroke,60 massive pulmonary embolism61–63 or prosthetic heart valve thrombosis64,65 treatment in pregnants, but no definitive data exist in this setting.
X-ray exposure during percutaneous treatment may have teratogenic repercussion on the foetus. However, it has been shown that by adopting the above mentioned precaution even time-consuming procedures result in a low exposure,55 with an overall 1.47 relative risk of developing malignant disease.48
Considering the different kind of pathogenetic mechanism of ACS in pregnants it is important to consider early how the amount of myocardium is at risk and rule out the aetiology. Furthermore it is necessary to tailor the optimal treatment. As a first-line strategy medical treatment and high intensive care units are reasonable. In case of pregnant patients a close interaction between cardiologist and gynaecologist is necessary to avoid a possible pharmacological adverse event both in mother and foetus.
Percutaneous Revascularisation
Angiographic pattern plays a key role in percutaneous versus surgical option. A careful evaluation of the aetiology, localisation and extension of the disease allows us to choose the best treatment strategy. Spasm and SCAD should be immediately ruled out. While AD management is already established in the literature, no clear SCAD percutaneous treatment is yet to be standardised. Some authors suggest to pre-dilate an occluding dissection while others prefer a direct stenting.21,66,67 In case of a proximal non-occlusive coronary artery SCAD we suggest direct stenting in order to seal the dissection and limit further proximal or distal propagation secondary to pre-dilatation. On the contrary, distal and non-flow limiting coronary artery SCAD should be carefully evaluated and a conservative watching and waiting strategy considered. On the other hand AD and SCAD may often be associated with coronary spasm and overlapped thrombus. Thus in case of completely occluded vessel it might be difficult to reveal the underling mechanism. In these situations we suggest to initially restore blood flow by means of conventional angioplasty by using an undersized balloon as the first choice. Mechanical aspiration devices and IVUS usage are left at the operator’s discretion while the intra-aortic balloon pump or left ventricular assist devices are recommended in cardiogenic shock. A careful evaluation of the number and length of the stents required is strongly suggested to avoid a ‘full metal jacket’ implantation. Always keep in mind that during the acute phase diffuse coronary artery spasm may occur and we consider frequent use of vasodilators as appropriate to limit stent implantation to the culprit lesion. Anecdotally control angiography distant from the index procedure in these patients has surprisingly shown better angiographic aspects of the non-culprit segments. This is important and should be kept in mind during the acute phase in order to identify the culprit lesion and avoid unnecessary treatment. A ‘keep it simple’ strategy is recommended when facing complex lesions, such as bifurcations trying to limit stent number by applying the concept of ‘provisional stenting technique’. No data exist on benefits and safety profile in DES implantation versus bare-metal stents. While in the post-partum period the choice of the stent type follows general consensus for percutaneous coronary interventions, in the pre-partum period it is very controversial. Finally, the young age and long-life expectancy of this cohort of patients should be considered for an optimal interventional treatment strategy.
Surgical revascularisation
Data regarding surgical revascularisation are almost lacking. Only a few cases reported the feasibility of surgery – mostly in the pre-partum patients.68 Similarly to the pharmacological and percutaneous strategies, surgery has different implications in pre- and post-partum women. In post-partum women indications for surgical revascularisation are based on general ACS guidelines and pattern of disease at angiography.49–52
In pre-partum women, cardiac surgery is hampered by high procedural risk. Foetal mortality ranges from 16 to 33 %.69 For this reason a conjunct decision with the gynaecologist should be obtained and contemporary caesarean delivery considered as appropriate during the last trimester.70,71
Conclusions
ACS in pre- and post-partum women is a rare but dreadful situation. Unless developments in diagnostic and therapeutic tools are made, mortality will remain high. It should be considered a multifactorial disease with a special role for sexual hormones. Prompt diagnosis of ACS and aetiology together with the risk stratification is mandatory. On this ground, tailored non-invasive/invasive or surgical therapy should be shared together with the gynaecologist, particularly in pre-partum women. Knowledge of drug effects in pregnancy and mastering revascularisation techniques and materials are pivotal to succeed in managing these complicated cases. Further progress in drugs and devices may be helpful in the near future to better address and treat these patients – i.e. new antiplatelet drugs72 or the advent of bioresorbable stents.73