Introduction
The ability of intra-coronary pressure and flow dynamics to determine stenosis severity was elegantly described by Gould more than 40 years ago.1 The potential to categorise stenoses into mild, moderate and severe by quantifying the combined effect of perfusion pressure, stenosis geometry and the amount of myocardium subtended provided the possibility of a more objective way of determining epicardial stenosis severity than visual estimation alone.
The following 20 years spawned several indices of stenosis severity that incorporated pressure (fractional flow reserve; FFR), flow (coronary flow reserve) and a combination of both parameters (hyperaemic stenosis resistance).
The most prominent clinical studies to determine the clinical utility of intracoronary physiology (specifically FFR) to guide intervention demonstrated that it was safe to defer intervention, and to guide intervention with FFR.2,3 These studies were in patients with stable coronary disease, and spanned several periods of intervention, including balloon-only angioplasty, bare-metal stents and first-generation drugeluting stents. The reduction in major adverse cardiac events (MACE) in the FFR arms of these studies was driven by lower rates of peri-procedural MI, which is consistent with there being less intervention in these patients.
As the benefit of this technology is appraised, it is natural for the community to test its utility in different patient populations. More recently, it has been tested in studies with a higher proportion of patients with acute coronary syndrome (ACS; FUTURE), ST-elevation MI (STEMI) or bystander disease (FLOWER), and to guide percutaneous coronary intervention (PCI) in patients with multi-vessel disease with outcomes compared to coronary artery bypass grafting (CABG; FAME 3).4–6 The results have been disappointing.
Does this mean physiology no longer has a role in guiding intervention? Do these studies point to a more restricted role for these indices in clinical practice? Or do these studies suggest a need for an index that provide a more comprehensive assessment of coronary physiology?
The following commentaries discuss the clinical utility of intra-coronary physiology (specifically FFR) in clinical practice in the context of the more recent clinical trials.
Modi and Dutta take the position that the wealth of data from the more contemporary studies and the limited information afforded from FFR regarding the microvasculature limits its utility in clinical practice. However, while Collison accepts these arguments, he points towards an evolution in how we use and interpret intracoronary physiology.
The limitations of FFR uncovered in these trials point to a need for indices that permit the assessment of the entire coronary vasculature. Indeed, while the recent studies of FFR have been less than convincing, they provide an impetus for the development and adoption of new indices of coronary physiology that also consider the effect of the microcirculation and its influence on cardiac events.
We are therefore at a crossroads with regards to intracoronary physiology. We can either relegate it to being a niche tool with limited clinical utility or we can realise the opportunity and insight afforded by the above studies and recognise the need to combine epicardial and microvascular information to categorise disease patterns across the entire coronary tree. Contemporary physiological assessment should be able to inform us about the epicardial and microvascular status of our patients. Whether we make this assessment using pressure-only indices or by combining both pressure and flow will be determined by the available technology and its ease of use. How we treat each patient cohort will require further trials.
The continuous re-evaluation of existing technology in clinical practice is vital for innovation and the evolution of any field. The above studies therefore do not mark the demise of intracoronary physiology as a tool to guide revascularisation but rather have refined our understanding of the limitations of existing indices and thereby ushered in the dawn of a new era.
Yes: For the Motion (Modi and Dutta)
Framing the debate in this way is perhaps a little misleading. A better question would be: “Has conventional use of the pressure wire to assess epicardial coronary disease had its day?”
Before the advent of physiology-guided revascularisation, operators relied primarily on their visual interpretation of the coronary angiogram, despite knowing it was merely a 2D representation of a 3D structure and that many more factors were influencing blood flow than luminal stenosis alone.7 This led to the development of fractional flow reserve (FFR), which enabled us to estimate blood flow impairment across a stenosis from a pressure gradient alone.
Following this, several trials showed the value of FFR-guided revascularisation against not only angiography-guided revascularisation but also medical therapy.8 This then led to the class 1 recommendations in clinical guidelines and appropriate use criteria in the US supporting pressure-wire guided revascularisation.9 However, in the past 3 years several trials have suggested that physiology-guided and ischaemiaguided revascularisation is perhaps not as important as we first thought.
Recent Negative FFR Trials
There have been several trials with negative data for FFR in recent times.
RIPCORD 2 was a trial of systematic pressure wire assessment of all epicardial vessels compared to angiography alone in patients presenting with both stable angina and non-ST segment elevation MI (NSTEMI). There was no significant difference between the two arms in the co-primary outcome related to costs and quality of life. The clinical composite hierarchical event (death, stroke, MI and unplanned revascularisation) rates were similar (8.7% for angiography alone versus 9.5% for systematic FFR; p=0.64).10
FLOWER MI was a multicentre trial where patients with STEMI and multivessel disease, after appropriate treatment of the infarct-related artery, were randomised to angiography versus FFR-guided further revascularisation of non-infarct-related arteries. FFR-guided revascularisation was not superior to angiography-guided revascularisation for the combined primary endpoint of death from any cause, non-fatal MI or urgent revascularisation. At 1 year, this rate was 5.5% in the FFR arm and 4.2% in the angiography-guided arm (p=0.31).4
FAME 3 was a trial of FFR-guided PCI versus coronary artery bypass grafting for three-vessel coronary artery disease. This was a trial of noninferiority, where PCI failed to show non-inferiority versus CABG with a combined outcome of all-cause mortality, MI, stroke and repeat revascularisation (10.6% in the FFR arm compared to 6.9% in the CABG arm; p=0.35)5
FUTURE was another trial where patients with stable angina and at least two significant coronary artery stenoses (>50% diameter stenosis) were randomised to FFR-guided versus traditional angiography-guided revascularisation. There was no significant difference in the primary endpoint of major adverse cardiac or cerebrovascular events rates of 14.6% in the FFR group versus 14.4% in the angiography-guided group (p=0.85). There was also no difference in all-cause mortality (3.7% versus 1.5%; p=0.06).6
Revascularisation for Stable Coronary Artery Disease in Decline
Historically, in stable coronary artery disease, angioplasty aimed to treat the symptoms of angina as well as provide a prognostic benefit regarding further cardiac events and mortality. It is in this setting where the pressure wire flourished and where most of its evidence base has accrued.
However, we are now beginning to question the prognostic value of angioplasty for stable coronary artery disease, with studies such as ISCHEMIA and COURAGE showing that an invasive strategy did not reduce ischaemic cardiovascular events or all-cause mortality when compared to optimal medical therapy.11,12 In addition, though we might assume there is a correlation between a trans-stenotic pressure drop and reduced perfusion leading to ischaemia, we have not seen evidence from major randomised controlled trials that patients with a high burden of ischaemia benefit from revascularisation.11
This has led to a decline in the use of PCI for stable angina patients, even those with ischaemia in a myocardial territory, unless there is a significant symptom burden resistant to antianginal therapy. Even in stable patients with cardiomyopathy due to significant coronary artery disease (CAD) with ischaemia and myocardial viability, the REVIVED trial has shown us that multivessel PCI gave no added mortality benefit over optimal medical therapy (37.2% in PCI group and 38% in the optimal medical therapy group; p=0.96).13
The consequence of these negative trials over the past decade is that less PCI is carried out in stable patients, even in the presence of myocardial viability or ischaemia. The British Cardiovascular Intervention Society’s national UK database for 2020–21 shows PCI is now predominantly undertaken in the setting of acute coronary syndromes (now 74.2% of all UK angioplasties compared to 45% in 2005). On the other hand, the percentage of PCI carried out in stable patients has decreased to 25.8%, compared to 2005 when it was above 50%.14
Why is that relevant to this debate? Well, the data underpinning the benefits of pressure wire assessment were accrued mainly in the setting of PCI for stable CAD. If we are doing less of that, this perhaps suggests we will need it less for epicardial coronary artery disease. As already discussed, we are also now seeing an increasing amount of evidence to suggest ischaemia is less important and data from trials such as SCOTHEART showing that plaque burden is perhaps a bigger driver of future events for patients with stable CAD.11
We have also seen studies on vulnerable plaque that have shown evidence of MACE to be independently associated with thin-cap fibroatheroma and plaque burden.15 FFR can generally be regarded as less able to predict plaque morphology than optical coherence tomography (OCT) or intravascular ultrasound (IVUS).
So, with declining use of PCI in stable patients, the increased awareness of the importance of plaque burden/morphology compared to ischaemia and the limited evidence base for the use of the pressure wire in ACS, the relevance of conventional pressure wire use is certainly up for debate.
Growth of Intracoronary Imaging
The use of intracoronary imaging to guide coronary interventions has been steadily rising in the UK over the past 10 years.14 Multiple studies, such as ULTIMATE and RENOVATE Complex PCI, have signalled that image-guided PCI has better outcomes than PCI guided by angiography alone.16,17 As intracoronary imaging-guided PCI grows regarding its evidence base and popularity, the natural question is: can you use it in place of the pressure wire to help identify flow-limiting coronary artery disease?
The correlation between intracoronary imaging minimum luminal area (MLA) values and FFR has always been subject to debate, with various cut-offs proposed for different populations. For example, the LITRO study showed in a Western population that an MLA value <6 mm2 correlates best with an FFR of <0.80 but Jang et al. showed in an Asian population that a much lower MLA of <4.8 mm2 correlates with an FFR of <0.80.18,19 Despite this, in practice, we commonly consider an MLA of <4 mm2 in nonleft main coronary artery epicardial vessels to correlate with an FFR of <0.80. This led to the FLAVOUR trial, which showed that IVUS-guided PCI was non-inferior to FFR-guided PCI when compared with outcomes of allcause mortality, myocardial infarction and repeat revascularisation at 24 months with rates of 8.1% in the FFR group and 8.5% in the IVUS arm (p=0.01).20
The increased use of intracoronary imaging to optimise PCI is clearly now being followed with a growing evidence base supporting its use as a surrogate of the pressure wire. In addition to this, a growing amount of work is being done in the field of intracoronary imaging-derived physiology using computation fluid dynamics. We will come onto this growing field later but the ability to truly identify the left main coronary artery has led to work to compute virtual FFR from it.
Ultrasonic flow ratio is a novel method for deriving FFR from IVUS and has shown a strong correlation with invasive FFR, independent of lesion location, with low inter- and intra-observer variability.21 OCT-based FFR computational approaches have also shown good correlation with invasive FFR with vessel-level OCT FFR being shown to be independent predictor of risk regarding target vessel failures in patients having PCI for ACS.22 Intracoronary imaging-derived FFR is clearly in its infancy but a field that will carry on growing.
Pressure Wire Best Used to Identify Pattern of Disease
As the value of ischaemia-guided PCI for stable CAD diminishes, a specific FFR/instantaneous wave-free ratio (iFR) threshold is now also in question. FFR and iFR are continuous biological variables for which various thresholds have been proposed.23 This concern has been growing for several years and has led to the pressure wire being increasingly used to identify patterns of disease that would respond best to PCI (i.e. focal rather than diffuse).
The TARGET-FFR study showed the value of post-PCI FFR measurements to optimise PCI and ensure a greater proportion of patients leave the catheter laboratory with an FFR >0.80.24 The use of the pressure wire has also been expanded to identify focal versus diffuse disease, with the development of the pressure pullback gradient (PPG) to quantify the degree of ‘diffuseness’: diffuse lesions lead to a lower PPG index and worse outcomes and symptom benefits from PCI.25
Growth of Computational Fluid Dynamics and Non-invasive Estimates of Physiology
The use of computational fluid dynamics (CFD) has revolutionised our ability to compute coronary artery flow from CT coronary angiography and angiography. CFD is a branch of fluid mechanics incorporating numerical analysis and data structures to assess fluid flows. This is then computed using mathematical calculations to simulate the free flow of fluid and its interaction with surfaces delineated by boundaries.
CT fractional flow reserve has led the way in this regard with data to support correlation with FFR and also to show its clinical value.26,27 More recently, angiography-derived FFR has entered the space with four major international vendors. Quantitative flow ratio (QFR; Medis) perhaps has the greatest evidence base with the FAVOR I, II and III studies showing high diagnostic accuracies of 97.7% for identifying haemodynamically significant coronary stenosis, and patient-level diagnostic accuracy was 92.4% (p<0.001 for both).28,29 Angiography-derived FFR indices all exhibit a similar degree of accuracy (QFR, FFRangio, coronary angiography-based wire-free FFR and vessel FFR).30 The technology is iteratively improving with a growing evidence base, and is likely to present a significant challenge to the conventional use of the pressure wire as time goes on.
Microvascular Assessment: The Saviour?
About two-thirds of patents have no significant CAD on angiography despite a convincing history of chest pain.31 We often overlook their symptoms but forget that a large part of the coronary tree is the coronary microvasculature, which cannot be seen on angiography.32
Studies such as CorMicA (which showed that a tiered approach for assessment for microvascular and/or vasospastic angina among patients with stable angina and no evidence of significant epicardial disease was superior to usual care) could enable us to improve angina and patient quality of life.33
The WISE trial before that showed the importance of assessing microvasculature in the female population as 33% of women recruited in the study had a death attributable to cardiac pathophysiology within 9 years of having an invasive angiogram that showed unobstructed coronaries.34
The index of microcirculatory resistance (IMR) and hyperaemic microvascular resistance are pressure wire-based indices of microvascular function and are growing in popularity as awareness of microvascular dysfunction grows. Even this area is a subject of CFD research, with coronary angiography-derived IMR being tested in a small cohort of patients.35
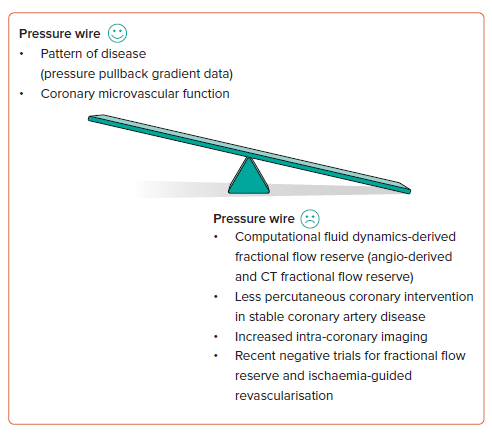
Summary
As we illustrate in Figure 1, PCI for stable CAD and ischaemia-guided PCI is falling, and the evidence for pressure-wire use in ACS is being questioned. Alongside this, we have seen several negative pressure wire studies together with a growth in intracoronary imaging both to guide PCI and as a surrogate for physiology. At the same time, we have seen an explosion in CFD-derived physiology, from both CT and angiography, with those technologies iteratively improving. Contemporary pressure-wire utility for epicardial CAD is evolving, so it is clearly a subject of debate and the reason we are having this discussion.
It is not all negative for the pressure wire. Its roles in identifying focal epicardial disease that responds best to PCI and in identifying coronary microvascular dysfunction are clearly growing. The pressure wire may not be dead but the way we use it is changing and the tide will be difficult to turn.
No: Against the Motion (Collison)
Rather than rush to retire the tried and trusted pressure wire, we should first look beyond the headlines from the recent slew of negative FFR trials and perform a more critical analysis of this data.
In RIPCORD 2, the authors randomised 1,100 patients undergoing invasive coronary angiography to receive either standard angiography-guided treatment (control group) or angiography plus systematic FFR assessments in all coronary arteries of sufficient calibre for PCI or placement of a bypass graft.10 At 1-year follow-up, there were no differences in costs, quality of life metrics or clinical events.
Performing FFR assessment in vessels where, based on the degree of angiographic stenosis, there is either a high or low probability of obtaining a value of ≤0.80 is unlikely to effect any significant change in initial treatment decisions – it will most likely just confirm the operator’s original impression from the angiogram. In cases of intermediate stenoses, however, FFR has the potential to change management plans; a negative FFR can reassure that deferral of PCI and continued medical therapy are appropriate whereas a positive FFR can confirm that a strategy of multivessel revascularisation is required.
In RIPCORD 2, however, more than two-thirds of all FFR measurements had a value of ≥0.80 and systematic multivessel FFR assessment reduced the proportion of patients with a plan for PCI from 61% to 56% (2% were deferred to medical therapy and 3% were escalated to CABG instead). In the FFR-guided group, investigators were able to declare a definitive management plan immediately after the procedure in >98% of patients whereas 14% of patients in the control group required an additional test. Of note, operators declared 20% of otherwise eligible and consented patients unsuitable for pressure wire assessment before randomisation and this potential selection bias is a limitation of the study.10 The message from RIPCORD 2 is not that FFR is ineffective but rather that a strategy of unselected, multivessel FFR assessment had no overall advantage compared to angiography alone.
FAME 3 randomised 1,500 patients with angiographically defined, three vessel CAD to undergo either CABG or FFR-guided PCI.5 With respect to a composite major adverse cardiac or cerebrovascular endpoint at 1 year, FFR-guided PCI did not meet the trial’s prespecified non-inferiority criteria compared to CABG. The expectation had been that FFR-guided PCI would avoid unnecessary stenting of non-flow limiting lesions, thereby preventing stent-related complications such as thrombosis or restenosis. FFR was measured in 82% of lesions (most of the remainder being subtotally or completely occluded vessels), of which 76% had an FFR ≤0.80. The incidence of non-ischaemic FFR values, and consequently PCI deferral rates, were lower than predicted so FFR could only possibly change treatment decisions in a small proportion of patients in this population. As other commentators have pointed out, FAME 3 essentially became another trial of PCI versus CABG in multivessel disease.
FLOWER-MI compared 1-year clinical outcomes between FFR and angiography-guided completion of revascularisation in 1,171 patients who had undergone successful infarct-related artery PCI for STEMI.4 FFR data were missing in 16% of lesions but, among those with available measurements, 44% had an FFR >0.80 which led to deferral of PCI. Overall, the rate of PCI on non-culprit lesions was 35% lower in the FFRguided arm. There was a slightly higher incidence of periprocedural MI in the FFR-guided arm (counterintuitive given less coronary intervention was performed in these patients), which was not statistically significant. The trial was underpowered due to lower than anticipated event rates but reported no difference in MACE between FFR-guided versus angiographyguided non-culprit revascularisation at 1 year. However, given the wide confidence intervals for the estimate of effect, the authors stated their findings did not allow for a conclusive interpretation.
The FUTURE trial had several limitations, and its results must be interpreted with some caveats.6 FFR de-escalated an initial PCI strategy to medical therapy in just 8% of cases (compared to 37% in the FAME trial). This will in part be because 11.5% of PCIs and 16.9% of CABGs were performed in vessels with an FFR >0.80 (thereby obviating the point of performing FFR). The trial was halted early due to an apparent signal for excess all-cause mortality in the FFR group, yet this was not borne out in the final analysis. The authors concluded that there was no difference in 1-year MACE rates between groups; however, the trial was underpowered and showed a perhaps implausibly large 30% relative risk reduction in the FFR arm.
Proponents of intracoronary imaging would no doubt highlight the low use of IVUS or OCT in these trials and perhaps suggest that the results would have been quite different had either of these modalities been employed instead of FFR. In the FLAVOUR trial, among patients with intermediate stenoses (mean SYNTAX score <10; baseline FFR 0.83±0.09) who were being evaluated for PCI, FFR guidance was non-inferior to IVUS guidance with respect to a composite primary outcome of death, MI or revascularisation at 24 months.20 The rate of PCI was 44% in the FFR arm and 65% with IVUS.
So where does that leave FFR? Rather than banging five nails into its coffin, the above trials have been quite informative in how best to use FFR. We should be selective; there is no overall benefit to assessing FFR in every vessel. Its utility lies in deferring unnecessary PCI so it will be most effective in assessing intermediate lesions of uncertain functional significance, particularly in the setting of stable angina. Fewer stents are implanted with FFR compared to PCI procedures guided by angiography or intracoronary imaging, and short-term clinical outcomes are reportedly no worse.
The question then remains: which is higher in the long run – the risk of spontaneous MI in an FFR-deferred vessel or the lifetime risk of stent thrombosis/restenosis from a stent placed in a vessel with functionally non-significant disease? Only large, well-designed and adequately powered trials with very long-term follow-up can answer that.
It has been proposed that study designs for diagnostic strategies should focus on discordant decisions (high FFR values do not usually alter angiography-based treatment decisions) and that composite endpoints for PCI should discard mortality and focus on vessel-level outcomes for spontaneous MI.36 Furthermore, if target vessel revascularisation (TVR) is to be considered a fair and valid metric to compare strategies with differing rates of PCI performance and deferral, the index PCI must also be included when calculating the total amount of TVR.36
FFR is far from receiving its last rites. It continues to evolve, and recent data support a progression away from the traditional stent/don’t stent decision based on a single point value in the distal vessel. Pressure wire pullbacks effectively provide us with a functional topographic map of an artery and new metrics such as the PPG further facilitate differentiation between focal and diffuse patterns of coronary disease. PCI achieves better functional results and improved patient-reported outcomes where it is applied to focal coronary artery disease.25,37 We need to move away from slavishly adhering to an artificial, dichotomous clinical cut-off value for FFR and place just as much importance on the pattern of coronary disease in a vessel when deciding on the appropriateness of PCI.
Finally, a word on the new kids on the block. Rather than representing an existential threat to the pressure wire, the advent of angiographyderived FFR is welcome if it expands access to coronary physiology assessment for patients being considered for PCI. However, outside clinical trials, its uptake in practice has been low to date and the trusty pressure wire is likely to remain the most widely available and used coronary physiology tool for some time to come. There is no need for a eulogy just yet.










