AF is a common rhythm disturbance. In Europe, the estimated prevalence of AF in 2010 was 8.8 million patients, which is projected to rise to 17.9 million patients in 2060, mostly due to the ageing of the population.1 Approximately 20–40% of AF patients present with coronary artery disease (CAD) or develop CAD over time.2 Conversely, approximately 5–10% of subjects referred for invasive coronary angiography have an established indication for oral anticoagulation (OAC), mainly due to AF.2
The vast majority of AF patients are at high risk of cardioembolic events, thus requiring long-term OAC for the prevention of stroke and extracranial systemic embolism.3 Conversely, CAD patients receive antiplatelet therapy to reduce the ischaemic burden and prevent acute events.4 In particular, a period of dual antiplatelet therapy (DAPT) is warranted for patients undergoing percutaneous coronary intervention (PCI) to minimise the risk of stent-related complications, including stent thrombosis.5
The overlap of AF and PCI implies challenges with regard to the management of antithrombotic therapy.6 Indeed, albeit theoretically indicated, long-term triple antithrombotic therapy (TAT) with OAC and DAPT is discouraged because of its detrimental effect on bleeding, which, in turn, affects patient prognosis.7 Advances in PCI and stent technology have enabled the use of less intense antithrombotic therapy, mitigating the bleeding risk without significant drawbacks in terms of thrombotic events.8–10
This review analyses the mechanisms underlying thrombotic complications in AF-PCI, summarises the evidence surrounding antithrombotic therapy management and comments on the latest European guidelines in this area.
Pathophysiological Premises
Antithrombotic drugs (i.e. antiplatelets and anticoagulants) targeting different processes (i.e. platelet activation and the coagulation cascade, respectively) can prevent thromboembolic complications.11 Although frequently intertwined, each pathway contributes to thrombus formation to a different extent, depending on underlying haemorheological circumstances and predisposing factors.12
Antithrombotic Therapy for AF
In AF patients, blood stasis and low shear stress in the left atrium, particularly in the left atrial appendage, trigger the coagulation cascade, leading to thrombus formation without a significant contribution from platelet activation.13 Accordingly, OAC is the preferred strategy to minimise the thrombotic burden and prevent cardioembolic stroke and systemic embolism.3 Nowadays, based on landmark trials in the field, direct oral anticoagulants (DOACs) are preferred over vitamin K antagonists (VKAs) for long-term OAC in AF patients without any specific contraindication.14–17
Early investigations demonstrated that aspirin is not as effective as VKAs in preventing cardioembolic stroke, and is associated with higher rates of ischaemic stroke in elderly AF patients.18,19 The ACTIVE-W trial, comparing OAC to DAPT in AF patients with additional risk factors for stroke, was prematurely stopped due to significantly reduced 1-year vascular events with OAC.20
In AF patients unsuitable for OAC, the ACTIVE-A randomised controlled trial (RCT) showed that DAPT reduced stroke and major vascular events while increasing major bleeding.21 More recently, the AVERROES trial showed that the DOAC apixaban outperformed aspirin in reducing stroke or systemic embolism, without significant increases in major bleeding and intracranial haemorrhage.22
Antithrombotic Therapy after Percutaneous Coronary Intervention
In PCI patients, platelet activation plays a central role in thrombus formation because of the high shear stress within the stented coronary segments.23 Therefore, DAPT represents the treatment of choice for preventing early stent-related complications, including stent thrombosis.24
Evidence from a number of RCTs demonstrates the superiority of antiplatelet therapy over OAC in PCI patients. An early trial showed that DAPT with aspirin and ticlopidine reduced the incidence of adverse cardiac events without any increase in severe bleeding versus OAC plus aspirin.25 Similarly, the FANTASTIC and MATTIS trials demonstrated lower rates of bleeding, vascular complications and stent occlusion with aspirin and ticlopidine compared with the combination of OAC and aspirin.26,27 Finally, the Stent Anticoagulation Restenosis Study randomised 1,653 PCI patients to aspirin alone, aspirin plus ticlopidine or aspirin plus VKA: DAPT resulted in lower rates of thrombotic events, without any significant difference in bleeding compared with aspirin plus VKA.28
Because more potent P2Y12 inhibitors (i.e. prasugrel and ticagrelor) and newer-generation drug-eluting stents have lowered the incidence of stent-related complications, contemporary investigations are mainly directed towards exploring strategies to decrease the impact of DAPT on bleeding.29
Combining Antithrombotic Drugs
The timing of thrombotic and bleeding complications differs in patients undergoing PCI: the risk of stroke or non-target-vessel myocardial infarction (MI) is steady, or potentially increases over time, whereas the risk of stent thrombosis is more predictable and highest in the first week after stent implantation.30–32 Bleeding events can be related to the access site or not, and show an early periprocedural incidence peak (mainly access site-related) and a subsequent steady risk over time, potentially influenced by antithrombotic therapy.33
Interestingly, antithrombotic therapy can be adjusted accordingly: in particular, following the initial more intense phase, the bleeding risk can be minimised by DAPT modulation strategies (i.e. shortening DAPT duration and switching to lower-potency regimens).34
Evidence at a Glance
The latest European recommendations on antithrombotic management in AF-PCI patients stemmed from six landmark trials (Supplementary Material Table 1 and Figure 1), including early investigations of VKA-based regimens and modern trials of DOAC-based dual antithrombotic therapy (DAT) versus VKA-based TAT.35–40
Antithrombotic Regimens in the Vitamin K Antagonist Era
The WOEST trial, which can be considered the pioneer of aspirin-free strategies, randomised 573 PCI patients on OAC to receive DAPT with aspirin and clopidogrel (i.e. TAT) or clopidogrel alone (i.e. DAT); at one year, DAT significantly reduced the incidence of bleeding (HR 0.36; 95% CI [0.26–0.50]; p<0.0001) compared with TAT.35 Interestingly, this finding was consistent in different subgroups, including patients with acute coronary syndrome (ACS).35 In addition, despite a lack of power for ischaemic outcomes, DAT outperformed TAT in reducing all-cause death (HR 0.39; 95% CI [0.16–0.93]; p=0.027) and major adverse cardiovascular events (HR 0.60; 95% CI [0.38–0.94]; p=0.025).35 Of note, DAT was superior to a very long TAT duration (i.e. one year), which is no longer standard of care.
The ISAR-TRIPLE trial investigated the effect of shortening TAT duration to 6 months or 6 weeks in 614 patients on OAC undergoing PCI.36 In that trial, 6-week TAT was not superior to 6-month TAT in terms of 9-month net clinical benefit (HR 1.14; 95% CI [0.68–1.91]; p=0.64).36 Not surprisingly, in the landmark analysis between 6 weeks and 9 months, bleeding was reduced with DAT compared with TAT (HR 0.68; 95% CI [0.47–0.98]; p=0.04).36
Randomised Trials of Direct Oral Anticoagulants
With the advent of DOACs, four RCTs investigated short durations of TAT followed by DOAC-based DAT in AF-PCI (Figure 1).37–40
The PIONEER AF-PCI trial enrolled 2,124 AF-PCI patients to compare three antithrombotic regimens:
- DAT with rivaroxaban 15 mg once daily plus a P2Y12 inhibitor;
- TAT with rivaroxaban 2.5 mg twice daily plus DAPT, followed by DAT with rivaroxaban 15 mg once daily and aspirin after P2Y12 inhibitor discontinuation; and
- TAT with VKA plus DAPT (control arm).
DAPT duration was established upfront and ranged from one to six or 12 months.37 The incidence of one-year clinically relevant bleeding was reduced in both rivaroxaban-based groups compared with standard TAT (rivaroxaban-based DAT: HR 0.59, 95% CI [0.47–0.76], p<0.001; rivaroxaban-based TAT: HR 0.63, 95% CI [0.50–0.80], p<0.001), and this finding was consistent across multiple subgroups.37 However, despite a lack of power for the assessment of ischaemic outcomes, the risk for stroke and stent thrombosis appeared to be numerically higher with both rivaroxaban-based regimens.
In the RE-DUAL PCI trial, 2,725 AF-PCI patients were randomly allocated to:
- DAT with dabigatran 110 mg twice daily plus a P2Y12 inhibitor;
- DAT with dabigatran 150 mg twice daily plus a P2Y12 inhibitor; or
- TAT with VKA plus a P2Y12 inhibitor and aspirin (for one-to-three months depending on stent type).38
Of note, DAT with dabigatran 150 mg was compared with a corresponding TAT group including patients who had been eligible for dabigatran 150 mg. With respect to major or clinically relevant non-major bleeding at a median follow-up of 14 months, DAT with dabigatran 110 mg was superior to TAT (HR 0.52; 95% CI [0.42–0.63]; p<0.001 for both non-inferiority and superiority), whereas DAT with dabigatran 150 mg was non-inferior to TAT (HR 0.72; 95% CI [0.58–0.88]; p<0.001 for non-inferiority).38 These results were consistent in subgroup analyses according to clinical presentation and type of P2Y12 inhibitor.41 In addition, the combination of both dabigatran groups was non-inferior to TAT with respect to the composite of death, MI, stroke, systemic embolism or unplanned revascularisation (HR 1.04; 95% CI [0.84–1.29]; p=0.005 for non-inferiority).38
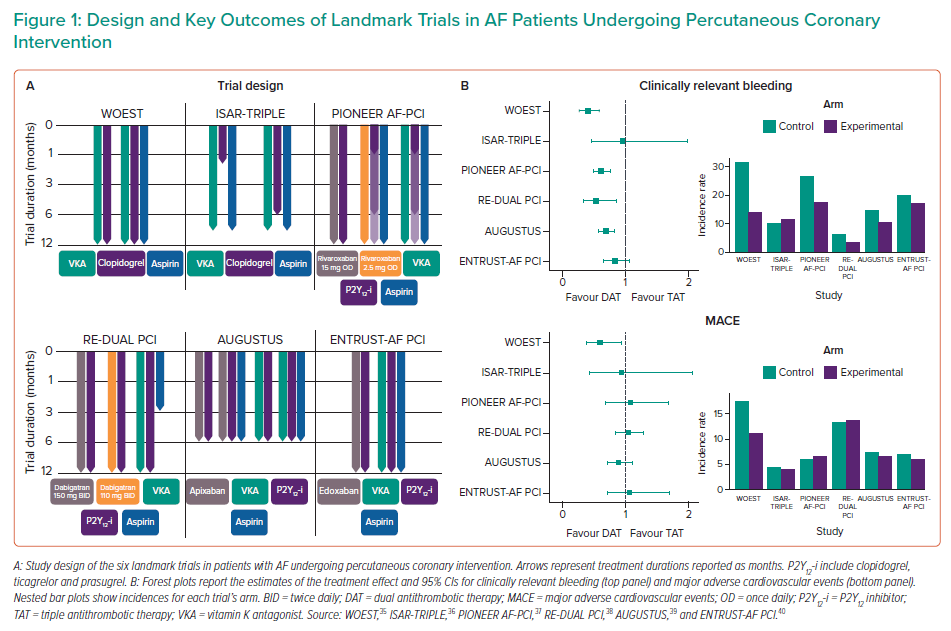
To discern benefits from the use of DOAC or aspirin withdrawal, the AUGUSTUS trial implemented an intriguing 2 × 2 factorial design: 4,614 AF patients undergoing PCI or with medically managed ACS who were planned to receive a P2Y12 inhibitor were randomised to receive either apixaban 5 mg twice daily or a VKA (open-label) and to receive either aspirin or placebo (blinded) for 6 months, thus resulting in four different regimens.39 No significant interaction was observed between the two randomisation factors with regard to any study outcome. At 6 months, apixaban reduced the incidence of major or clinically relevant non-major bleeding (HR 0.69; 95% CI [0.58–0.81]; p<0.001 for both non-inferiority and superiority) and the composite of death or hospitalisation (HR 0.83; 95% CI [0.74–0.93]; p=0.002), without any difference in the composite ischaemic endpoint (HR 0.93; 95% CI [0.75–1.16]) compared with VKA; interestingly, apixaban reduced the incidence of stroke compared to VKA (HR 0.50; 95% CI [0.26–0.97]).39 In the comparison of antiplatelet regimens, dropping aspirin reduced the incidence of major or clinically relevant non-major bleeding (HR 1.89; 95% CI [1.59–2.24]; p<0.001) without a concomitant increase in death or hospitalisations (HR 1.08; 95% CI [0.96–1.21]) or in ischaemic events (HR 0.89; 95% CI [0.71–1.11]).39 The AUGUSTUS trial disentangled the relative contributions of DOAC and dropping aspirin to reductions in bleeding, showing that beneficial effects derive from both these strategies.39 However, the effects of aspirin withdrawal should be interpreted in the light of several considerations: first, a short TAT was also administered to patients randomised to placebo (median time from PCI to randomisation 6 days); second, although the trial was underpowered to assess ischaemic outcomes, there were numerically increased rates of MI and stent thrombosis among patients receiving placebo compared with those receiving aspirin; third, the follow-up was shorter than in previous investigations. Interestingly, a subanalysis of the AUGUSTUS trial highlighted that benefit from TAT was relevant in the early post-PCI period, but decreased thereafter and was outweighed by the risk of severe bleeding after 30 days.42
In the ENTRUST-AF PCI trial, 1,506 patients were randomised to receive edoxaban 60 mg once daily plus a P2Y12 inhibitor for 12 months or TAT with a VKA, a P2Y12 inhibitor and aspirin (for 1 to 12 months).40 This trial showed non-inferiority, but not superiority, of edoxaban-based DAT compared with TAT in terms of 1 year major or clinically relevant non-major bleeding (HR 0.83; 95% CI [0.65–1.05]; p=0.001 for non-inferiority and p=0.115 for superiority), without any between-group difference in the composite efficacy outcome (HR 1.06; 95% CI [0.71–1.69]).40
Pooled Evidence and Ischaemic Outcomes
The above-mentioned RCTs were designed to strengthen DAT as the strategy of choice for the reduction of bleeding in AF-PCI patients. However, notwithstanding a consistent direction towards preserved efficacy with DAT regimens, none of the studies of individual DOAC-based strategies was powered to assess ischaemic outcomes.
Pooling evidence from RCTs, meta-analyses were conducted to explore whether DAT was associated with detrimental effects in terms of ischaemic events as opposed to TAT. These studies raised concerns about higher rates of stent thrombosis with DAT compared with TAT.43–48 However, using data from the DAT versus TAT comparison in the AUGUSTUS trial may have confounded the results. A subsequent Bayesian meta-analysis incorporated data about stent thrombosis from the AUGUSTUS trial for the specific comparison of apixaban-based DAT and VKA-based TAT.49 That analysis showed that there was no statistically significant difference in stent thrombosis with DOAC-based DAT compared with VKA-based TAT (HR 1.38; 95% CI [0.86–2.20]), particularly when DAT with dabigatran 110 mg was excluded from the analysis (HR 1.22; 95% CI [0.74–2.03]).50
Long-Term Antithrombotic Therapy
Two RCTs investigated long-term antithrombotic therapy for AF patients with chronic coronary syndrome (CCS).51,52 The OAC-ALONE non-inferiority trial randomly compared OAC (either VKA or DOAC) and DAT (OAC plus aspirin or clopidogrel) as long-term strategies beyond 1 year after PCI. Being prematurely terminated due to slow enrolment, that trial failed to demonstrate the non-inferiority of OAC alone to DAT in terms of the composite of all-cause death, MI, stroke, or systemic embolism at one year.51
The AFIRE trial compared rivaroxaban monotherapy and DAT with rivaroxaban in AF patients who had undergone myocardial revascularisation more than one year before or with angiographically diagnosed CAD.52 The trial was stopped early because of increased mortality in the DAT group; at a median follow-up of 24 months, rivaroxaban monotherapy was non-inferior to DAT for ischaemic events (HR 0.72; 95% CI [0.55–0.95]; p<0.001 for non-inferiority) and superior for bleeding (HR 0.59; 95% CI [0.39–0.89]; p=0.01 for superiority).52
These RCTs should be interpreted in light of several considerations potentially limiting their external validity: both RCTs were conducted in East Asian patients, who are well known to be more prone to bleeding than Western patients.53 In addition, the most represented OAC in the OAC-ALONE trial was VKA, and rivaroxaban doses in the AFIRE trial were not those approved in Europe for stroke prevention in AF.
Guidelines and Practical Management
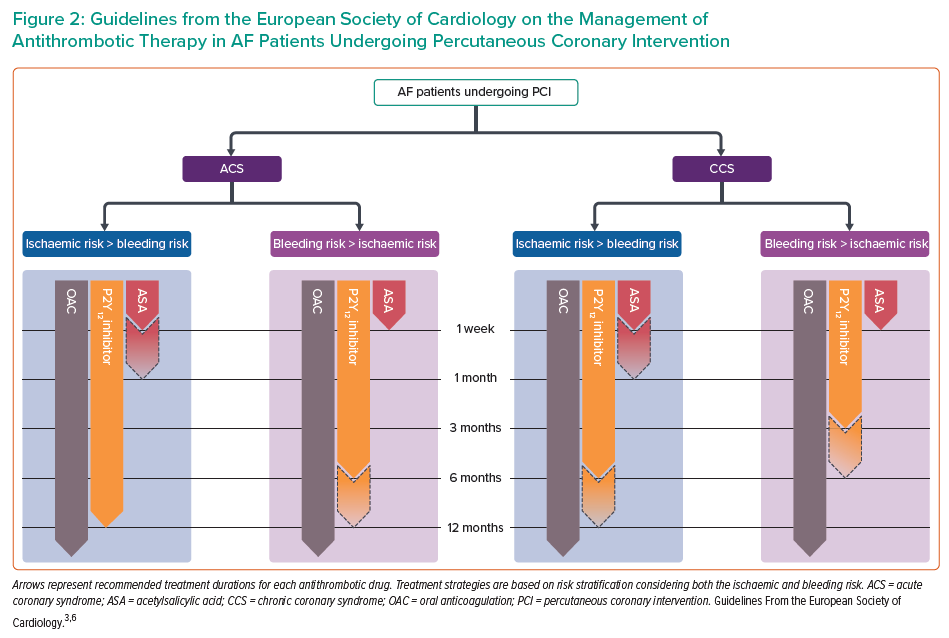
The latest guidelines from the European Society of Cardiology (ESC) provide updated recommendations concerning antithrombotic therapy management in AF-PCI patients (Figure 2).3,6
Oral Anticoagulant of Choice
As recommended by current European guidelines, in the absence of specific contraindications, a DOAC must be preferred over VKAs, regardless of concomitant antiplatelet therapy (class of recommendation [COR] I, level of evidence [LOE] A).3,6
This recommendation is based on the observation of a class effect with DOACs, which proved to be similarly efficacious and safer than VKAs.54 In addition, VKA therapy comes with practical challenges, including maintenance of target international normalised ratio (INR) and time in therapeutic range. In this regard, switching from a VKA to a DOAC can represent a promising bleeding-avoidance strategy in selected patients; interestingly, a RE-DUAL PCI subanalysis demonstrated a lower bleeding risk with dabigatran-based DAT compared with VKA-based TAT, regardless of prior OAC.55
However, not all subjects are eligible for DOACs, and VKAs remain the standard OAC for patients with moderate-to-severe mitral stenosis or mechanical valve prostheses.3
Choosing a Direct Oral Anticoagulant
All DOACs have shown good performance in reducing bleeding without any drawback in ischaemic events compared with VKAs, but no head-to-head RCT has ever been conducted. Thus, the choice of one DOAC over another should be essentially informed by individual patient characteristics (i.e. renal function, concomitant drugs, coexisting conditions).
Dosing Direct Oral Anticoagulants
As recommended by European guidelines, DOACs in TAT should be used at the approved doses for the prevention of stroke in AF.3,6 Doses should be reduced according to package labels when conditions potentially influencing drug metabolism and the risk of bleeding (e.g. renal failure, older age, concomitant drugs) coexist.3,6 To minimise bleeding risk, dabigatran 110 mg and rivaroxaban 15 mg should be preferred over their full doses (i.e. dabigatran 150 mg, rivaroxaban 20 mg) for the duration of concomitant antiplatelet therapy (COR IIa, LOE B).3,6
Optimal Duration of Triple Antithrombotic Therapy
Despite European guidelines supporting a default DAT strategy, a short course of TAT is necessary as protection for the periprocedural period (in-hospital, up to one week).3,6
Acute Coronary Syndrome
In ACS patients with an established long-term indication for OAC, a short periprocedural TAT with aspirin and clopidogrel on top of OAC is recommended (COR I, LOE A), followed by DAT (with an antiplatelet agent, preferably clopidogrel, and a DOAC) for up to 12 months (COR I, LOE A).6 In patients at high bleeding risk (HBR) with a low risk of stent thrombosis, the guidelines recommend discontinuation of TAT after 1 week and continuation of DAT with an OAC and a P2Y12 inhibitor (preferably clopidogrel) for up to 12 months (COR I, LOE A).3,6 Conversely, if concerns about stent thrombosis outweigh the bleeding risk, a longer duration of TAT (up to 1 month) should be considered (COR IIa, LOE C).3,6 Although prasugrel and ticagrelor are not recommended for TAT (COR III, LOE C), a DAT including one of these drugs is an alternative to standard TAT in patients at moderate-to-high risk of stent thrombosis (COR IIb, LOE C).6 Notably, a subanalysis of the RE-DUAL PCI trial showed that the benefit of DAT with dabigatran over TAT with a VKA in reducing bleeding risk was consistent across patients on ticagrelor or clopidogrel.56 No similar data are available for prasugrel, translating into a lack of supporting evidence also in the setting of DAT.
Chronic Coronary Syndrome
In CCS patients, the durations of TAT and DAT also vary depending on the individual risk profile, which includes patient characteristics, clinical presentation and angiographic features. In particular, if the risk of stent thrombosis is low or if concerns about bleeding prevail, early cessation of aspirin (within 1 week) and continuation of DAT for up to 6 months are recommended, regardless of the stent type (COR I, LOE A).3 Conversely, a longer TAT (up to 1 month) should be considered when the risk of stent thrombosis outweighs the bleeding risk (COR IIa, LOE C).3
Moving from Triple to Dual Antithrombotic Therapy
The switch from TAT to DAT can theoretically be made by either withdrawal of aspirin or discontinuation of the P2Y12 inhibitor. The former strategy is usually preferred due to its broader adoption in landmark AF-PCI trials, although both strategies have been investigated in patients with or without an indication for OAC.57–59 In addition, evidence is accruing on the long-term comparison of monotherapies in PCI patients not requiring OAC, with clopidogrel outperforming aspirin in the prevention of net adverse cardiovascular events.60 Further research is needed to confirm the applicability of these findings in the setting of AF-PCI patients.
Platelet function and genetic testing have the potential to identify subjects who are at higher risk for ischaemic or bleeding complications with clopidogrel.61 Although these tools are currently recommended for guided DAPT de-escalation in ACS (COR IIb, LOE A), their adoption could also be explored to guide and personalise the choice of the switching modality from TAT to DAT.6
P2Y12 Inhibitor of Choice
Whether more potent P2Y12 inhibitors (i.e. prasugrel and ticagrelor) can be used in DAT or TAT regimens is an interesting question. In the PIONEER
AF-PCI trial, prasugrel and ticagrelor were allowed but poorly used (5.6% of the total population).37 Ticagrelor was administered to approximately 12% of the RE-DUAL PCI patients and, although there was no statistical interaction between the treatment effect of the two doses of dabigatran and the choice of P2Y12 inhibitor, TAT with ticagrelor increased the absolute rates of bleeding.56,62 The use of ticagrelor and prasugrel was also low in the AUGUSTUS (6.2%) and ENTRUST-AF PCI (7.6%) trials.39,40 Based on these data and on the observation of high bleeding rates with ticagrelor and prasugrel in TAT regimens, current guidelines discourage the use of ticagrelor or prasugrel in this setting.6
Long-term Monotherapy
After 3–12 months (depending on the clinical scenario and the patient’s risk profile), OAC alone is recommended as long-term antithrombotic therapy.3,6 Registry data support this recommendation, whereas alternative strategies have been discouraged by inconclusive RCTs.51,52,63–66 However, because CAD patients maintain a high risk of long-term adverse cardiovascular events, further investigations are warranted.
North American Perspective
Recommendations on antithrombotic regimens and their durations are similar on both sides of the Atlantic.67,68 A slight difference can be noted in CCS patients without features of HBR, whereby North American guidelines suggest that a short period of TAT is followed by a 6-month DAT with a P2Y12 inhibitor (preferably clopidogrel), after which either a P2Y12 inhibitor or aspirin can be administered on top of OAC for up to 12 months.68
Risk Stratification
Risk stratification by means of the CHA2DS2-VASc score (congestive heart failure, hypertension, age, diabetes, stroke or transient ischaemic attack, vascular disease, sex) is of key importance to determine whether an AF patient requires long-term OAC, which is indicated by a CHA2DS2-VASc score ≥1 in men and ≥2 in women.3
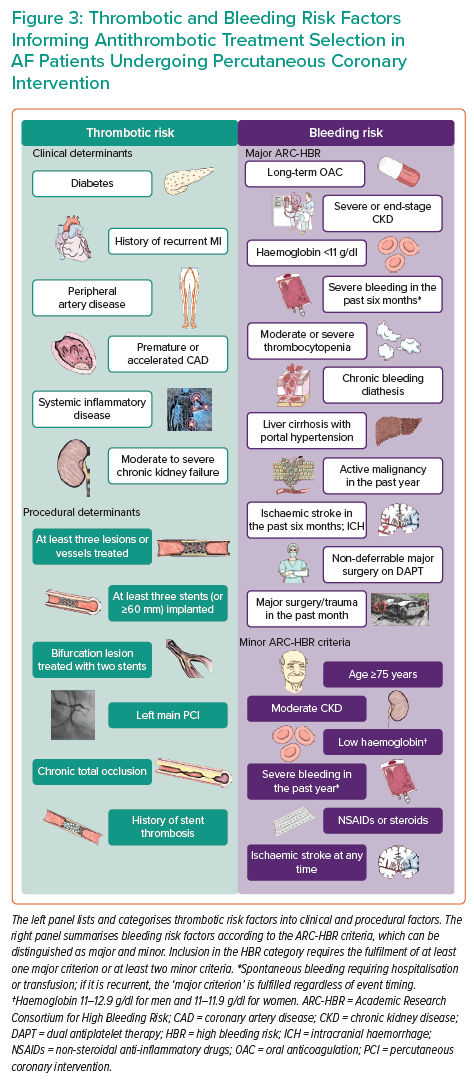
Based on such indications and the evidence stemming from RCTs, DAT is the regimen of choice for most AF-PCI patients because, compared with TAT, it mitigates the bleeding risk while effectively preventing ischaemic events. However, several risk factors and comorbidities contribute to one or both of the ischaemic and bleeding risks, making a comprehensive assessment necessary to maximise the benefit of antithrombotic therapy (Figure 3).69
A suggested approach by European guidelines is to calculate and weigh separate risk scores for ischaemia and bleeding one over another.70 The risk of ischaemic events is usually estimated by considering clinical, anatomical and procedural features, as well as by calculating specific risk scores, such as the GRACE score for ACS patients.71,72 Conversely, HBR patients are identified by a HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly) score ≥3, a PRECISE-DAPT score ≥25 or the fulfilment of at least one major or two minor Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria.73–76 Interestingly, the performance of the ARC-HBR model was found to be lower in ACS than CCS because ACS presentation was a strong predictor of bleeding per se.77 In addition, the ARC-HBR trade-off model was developed and was made available as a smartphone application to weigh the opposite and intertwined risks for ischaemic and bleeding complications at the individual level, thus informing the choice of optimal antithrombotic therapy.78
Subsets of Interest
Subgroup analyses of RCTs are available, helping to define the net benefit of antithrombotic strategies in specific contexts.
Age
In a subanalysis of the RE-DUAL PCI trial, dabigatran 110 mg DAT reduced bleeding compared with TAT in patients older than 75 years of age and even more in younger patients (p for interaction=0.013), whereas dabigatran 150 mg DAT only reduced bleeding in younger subjects (HR 0.57; 95% CI [0.44–0.74]; p for interaction=0.001).79 With regard to ischaemic outcomes, there were signals for higher risk with dabigatran 110 mg DAT compared with TAT in older (HR 1.54; 95% CI [1.07–2.22]) but not younger patients (p for interaction=0.029). In contrast, ischaemic events with dabigatran 150 mg DAT compared with TAT were similar in both older and younger patients (p for interaction=0.129).79
Race
RCTs informing current European guidelines included mostly or exclusively Western patients, thus limiting the generalisability of study results to other racial groups, including African American or East Asian patients. This restriction should be taken into account to prompt race-specific considerations based on major differences in the tendencies of different racial subgroups towards thrombotic or bleeding events.80
Diabetes
A subanalysis from the RE-DUAL PCI trial confirmed the benefit of dabigatran 110 mg DAT over TAT in diabetic patients in terms of bleeding reduction, with comparable rates of ischaemic outcomes.81 However, DAT with dabigatran 150 mg showed similar rates of bleeding and ischaemic outcomes as TAT in diabetic patients.81
Chronic Kidney Disease
Patients with renal failure are at high risk of both ischaemic and bleeding complications. In a subanalysis of the RE-DUAL PCI trial, DAT with dabigatran (either 110 or 150 mg) reduced bleeding complications compared with TAT, regardless of creatinine clearance (p for interaction=0.19 and 0.31, respectively).82 In terms of thromboembolic events, compared to TAT there was no difference with DAT with dabigatran 110 mg (p for interaction=0.30), whereas DAT with dabigatran 150 mg was shown to be beneficial in patients with normal renal function (p for interaction=0.02).82
In an AUGUSTUS subanalysis, apixaban was superior to VKA in decreasing both bleeding and ischaemic outcomes, with greater benefits with regard to bleeding in patients with a creatinine clearance between 30 and 50 ml/min.83 Conversely, there was no difference between aspirin and placebo in terms of ischaemic events, but higher rates of bleeding in patients on aspirin, particularly in those with preserved renal function (p for interaction=0.007).83 Of note, creatinine clearance <30 ml/min was an exclusion criterion in the AUGUSTUS trial.
High Bleeding Risk
ARC-HBR criteria have been recently validated in contemporary cohorts, and a trade-off model was developed to simultaneously derive and balance the bleeding and thrombotic risks in HBR patients.76,78,84–86 Although the ARC-HBR criteria are not specific for AF-PCI patients, it should be noted that most AF patients fulfil a major criterion of the ARC-HBR definition (i.e. anticipated use of long-term OAC).
The PRECISE-DAPT score was applied to a cohort from the RE-DUAL PCI trial: compared with TAT, DAT with dabigatran 110 mg reduced the risk of bleeding in both HBR and non-HBR patients, with the latter benefitting more from this regimen (p for interaction=0.02).87 DAT with dabigatran 150 mg significantly reduced bleeding only in non-HBR patients (HR 0.60; 95% CI [0.45–0.80]; p for interaction=0.08). No between-groups difference was noted in terms of ischaemic events in both HBR and non-HBR patients (p for interaction=0.45 and 0.56 for dabigatran 110 and 150 mg, respectively).87
Recently, the MASTER-DAPT trial enrolled 4,434 HBR patients undergoing PCI and demonstrated non-inferiority of 1-month DAPT to standard DAPT (at least 3 months) with regard to net adverse clinical events and major adverse cardiac or cerebral events, with better outcomes in terms of major or clinically relevant non-major bleeding.58 In the MASTER-DAPT trial, the randomisation was stratified by prior indication to OAC and a subgroup analysis showed that there was no difference in net benefit and ischaemic outcomes between short and standard DAPT in both patients with or without OAC indication (p for interaction=0.35 and 0.45, respectively). However, although short DAPT was associated with lower bleeding rates in patients without OAC, this difference was no longer evident in OAC patients.88
Beyond optimal antithrombotic therapy selection, HBR patients should be targeted with concomitant interventions to mitigate the bleeding risk (i.e. bleeding-avoidance strategies), including the use of proton pump inhibitors, avoidance of routine antiplatelet pretreatment and implementation of periprocedural measures (e.g. radial access and appropriate stent selection).89 In addition, non-pharmacological strategies, such as left atrial appendage occlusion, could be considered valuable options for HBR patients.90
Acute Coronary Syndrome
Clinical presentation has been investigated as a factor potentially influencing the relative efficacy and safety of antithrombotic regimens. In particular, several limitations in the prevention of ischaemic events with DAT have been hypothesised in ACS patients.
In a prespecified subgroup analysis of the RE-DUAL PCI trial, both dabigatran DAT regimens proved superior to TAT in reducing the bleeding risk in patients with or without ACS, regardless of the P2Y12 inhibitor used.56 However, although the trial was not powered for thrombotic outcomes and there was no statistically significant interaction between clinical presentation and outcomes, DAT with dabigatran 110 mg was associated with higher risks of MI (HR 1.87; 95% CI [1.02–3.40]) and stent thrombosis (HR 3.76; 95% CI [1.06–13.31]) in ACS patients.56 Consistently, a RE-DUAL PCI subanalysis of patients presenting with ST-segment elevation MI showed that DAT with dabigatran 110 and 150 mg outperformed TAT in lowering the incidence of bleeding (p for interaction=0.31 and 0.16) without negatively affecting the risk of thromboembolic events (p for interaction=0.20 and 0.33, respectively).41
An AUGUSTUS subgroup analysis of different clinical scenarios (i.e. medically managed ACS, ACS undergoing PCI and elective PCI) showed that DAT reduced the incidence of bleeding (p for interaction=0.479) and maintained a similar efficacy in the prevention of ischaemic outcomes (p for interaction=0.710) compared with TAT in ACS patients, whether undergoing PCI or not.91
A prespecified subanalysis of the ENTRUST AF-PCI trial showed that edoxaban-based DAT was associated with similar rates of bleeding and ischaemic events regardless of clinical presentation (p for interaction=0.274); in ACS patients, DAT was not associated with different risks of bleeding and thrombotic events compared with TAT.92
Complex Percutaneous Coronary Intervention
A PIONEER AF-PCI subanalysis investigated the interaction between procedural features and clinical outcomes and showed no effect modification by procedure or lesion characteristics for either clinically significant bleeding or major adverse cardiovascular events.93
In a post hoc analysis of the RE-DUAL PCI trial, both dabigatran 110 mg and 150 mg DAT regimens reduced the bleeding risk compared with TAT (p for interaction=0.90 and 0.37, respectively) without affecting thromboembolic outcomes (p for interaction=0.67 and 0.54, respectively), regardless of procedural complexity.94
Future Directions
Numerous trials are currently exploring antithrombotic management in AF-PCI (Supplementary Material Table 2). In the ACS setting, the focus is mainly directed towards appraising the role of combinations of DOACs and more potent P2Y12 inhibitors. The ADONIS-PCI non-inferiority RCT (NCT04695106) is enrolling AF patients with ACS to compare DAT with dabigatran (150 or 110 mg twice daily) and ticagrelor (90 mg twice daily for 1 month, followed by 60 mg twice daily for up to 12 months) to TAT with dabigatran, clopidogrel and aspirin followed by DAT (timing dependent on the bleeding and ischaemic risks) in terms of 2-year major or clinically relevant non-major bleeding.
The OPTIMA-3, 4 study (NCT03234114) contains two substudies: Chinese AF-PCI patients arbitrarily choose to receive a VKA or dabigatran, and are enrolled into the OPTIMA-3 or OPTIMA-4 substudy, respectively. The OPTIMA-3 study explores different TAT durations (1 versus 6 months) for the prevention of ischaemic events. The OPTIMA-4 study randomises patients on dabigatran 110 mg twice daily to ticagrelor 90 mg twice daily or clopidogrel 75 mg once daily for 12 months, and the two groups will be compared in terms of both 1-year major or clinically relevant non-major bleeding and major adverse cardiovascular events.
To explore the benefits of an early intensive antithrombotic regimen and subsequent de-escalation, the EPIDAURUS trial (NCT04981041) randomises AF-ACS patients to receive DAT with a DOAC plus prasugrel or ticagrelor for 1 month, followed by standard DAT (clopidogrel plus DOAC), or standard DAT upfront; the comparison will be made at 6 months in terms of both clinically relevant bleeding and major adverse cardiovascular events. Moreover, the APPROACH-ACS-AF trial (NCT02789917) is randomly investigating the role of DAT with apixaban 5 mg twice daily plus clopidogrel versus standard TAT with VKA.
In the PCI setting (mixed CCS and ACS), the COACH-AF-PCI trial (NCT03536611) is randomising AF-PCI East Asian patients to receive dabigatran 110 mg twice daily or a VKA on top of DAPT with aspirin and clopidogrel; aspirin is withdrawn at 1 month in both study groups and DAT is then administered for at least 5 months. The primary endpoint will be 2-year major bleeding.
Interestingly, the WOEST-3 RCT (NCT04436978) is testing an alternative strategy to reduce early stent-related complications in AF-PCI patients, who are randomised to standard TAT or DAPT (aspirin plus clopidogrel) for the first month, both followed by DAT for up to 1 year. The primary endpoint will be 1-month major or clinically relevant non-major bleeding.
Finally, two RCTs are investigating alternative strategies for AF-PCI patients over the long term. The ADAPT-AF RCT (NCT04250116) is comparing the long-term effects of DAT (apixaban 5 mg twice daily or rivaroxaban 15 mg once daily plus clopidogrel) or OAC alone (either DOAC or a VKA) on 2 year net adverse clinical events in AF patients who underwent PCI more than 1 year before. The AQUATIC trial (NCT04217447) will randomise approximately 2,000 AF patients who underwent PCI more than one year before receiving aspirin or placebo on top of OAC (either a VKA or DOAC) to assess the impact of these approaches on long-term major adverse cardiovascular events and bleeding.
Conclusion
Beyond periprocedural aspirin, when an established long-term indication for OAC coexists, PCI patients require a DAT, which increases the bleeding risk and is not an option over the long term. RCTs have demonstrated that DAT significantly reduces bleeding complications, without any apparent drawback in terms of ischaemic events. Accordingly, European guidelines recommend a very short duration of TAT (in-hospital or up to one week) followed by DAT with a DOAC and a P2Y12 inhibitor, the duration of which depends on the individual patient’s thrombotic and bleeding risk profiles. However, by investigating the role of more potent drugs and recent advances in the PCI field, future RCTs will shed more light on the optimal antithrombotic therapy management for AF-PCI patients.
Click here to view Supplementary Material.
Clinical Perspective
- AF and CAD often coexist, thus requiring a combination of anticoagulants and antiplatelet agents to prevent thrombotic complications.
- Stacking antithrombotic drugs increases the risk of bleeding; the latest European guidelines yielded several recommendations (e.g. on drug selection, TAT duration) to optimise the net benefit of antithrombotic therapy.
- Antithrombotic therapy should be tailored to individual patients; risk stratification is key in assessing and balancing individual ischaemic and bleeding risk profiles.
- Several patient subsets are at high risk of both ischaemic and bleeding complications (e.g. elderly, patients with chronic kidney disease, patients with HBR features undergoing complex coronary intervention), thus making antithrombotic therapy management an even more complex clinical challenge.
- Notwithstanding the growing evidence in the field, further data are needed to investigate new strategies directed at improving outcomes of AF patients undergoing PCI.










