A bifurcation lesion within the coronary arterial circulation is defined as a stenosis occurring at, or adjacent to, a significant division of a major epicardial coronary artery.1 Bifurcation lesions account for 1–20 % of all percutaneous coronary interventions (PCIs), and treatment remain technically challenging despite advances in PCI techniques and third-generation drug-eluting stent (DES) technology. In general, outcomes are less favourable, with an increase in the rates of major adverse cardiac events (MACE), target lesion revascularisation (TLR) and target vessel revascularisation.2,3
Recommendations from the European Bifurcation Club (EBC)1 derived from a systematic evaluation of over 10 years of data comparing a provisional single stent (main vessel [MV] only) with a planned two stent (MV plus side branch [SB]) conclude that a provisional single-stent approach should be the technique of choice for the treatment of the majority of bifurcation lesions. If SB coverage is then required, either a T or T and protrude (TAP) technique can be adopted to secure Thrombolysis in Myocardial Infarction flow.1 Favourable results with this technique, however, is dependent on successful SB rewiring, which may be technically challenging, particularly when SB vessels contain significant ostial disease. Difficulties may also be encountered when treating disease in the distal MV once the SB has been treated with TAP configuration and especially with a long neocarina protruding into the MV.
Despite their overall recommendations, the EBC concede that there will always remain a role for an upfront two-stent strategy covering both MV and SB.1 There are a number of two-stent strategies available, as outlined in the comprehensive review by Louvard et al.4 None of these have demonstrated any benefit over provisional MV stenting in the randomised trials, with the exception of the double kiss (DK) crush technique.5 Despite this, MV and SB two-stent techniques continue to be performed by PCI operators largely because the SB disease is considered to be causative of ischaemia or the SB would be significantly compromised by an MV-only stent strategy and needs to be protected first with a stent.
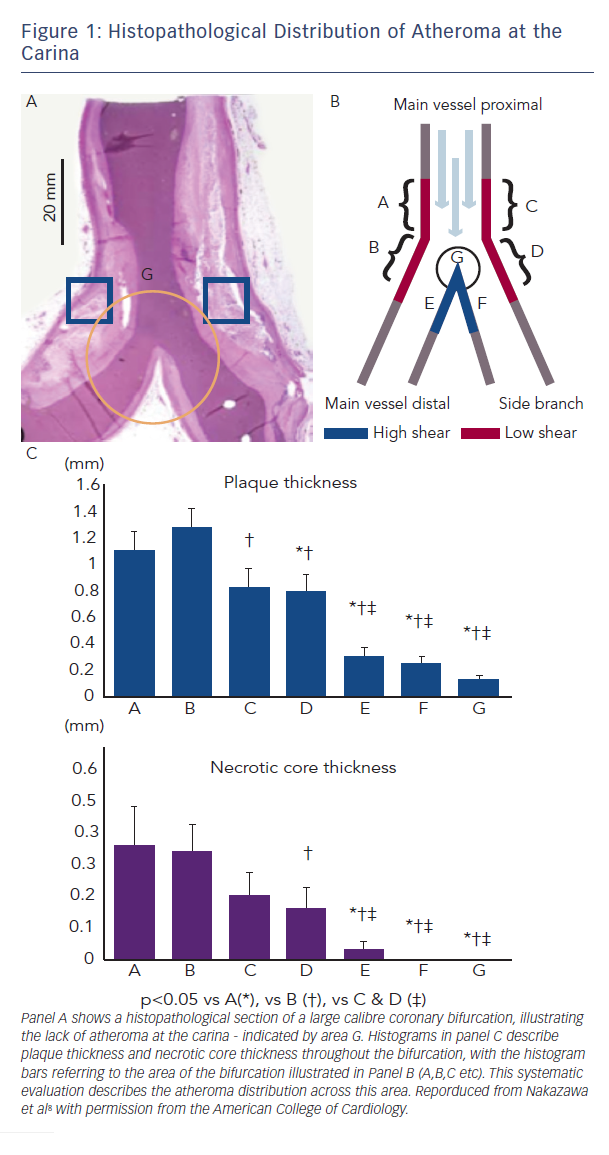
To be certain of ostial coverage, all two-stent bifurcation techniques (crush/TAP/culotte etc) result in neocarina formation, with multiple metallic stent layers overlapping. Compounding this is the inherent geometric disparity between the Y-shape of the bifurcation and the cylindrical tube of an expanded stent.6 This can delay healing, with reduced intima formation over these sites, which may explain the higher incidence of procedural myocardial infarction (MI), stent thrombosis and restenosis that has been observed with such techniques.7 The carina itself is subject to high shear stress and flow, and hence often free from significant atheroma. Systematic histopathological analysis has confirmed this, questioning the benefit of covering this area with metal (Figure 1).8 An interventional technique that avoids creation of a neocarina, leaving it ‘metal free’ and facilitating SB access may, therefore, offer advantages in the treatment of complex bifurcation lesions.
One solution to these issues is the dedicated AXXESS™ bifurcation stent system (Biosensors Interventional Technologies, Singapore). This follows a provisional strategy while offering predictable access to the SB and MV in the long term, with the absence of metallic layers at the carina. The aim of this article is to describe the AXXESS system and the techniques employed at implantation, and to summarise the growing evidence base to support its use in the treatment of complex coronary bifurcation lesions.
The AXXESS Stent
The Biosensors AXXESS Biolimus A9™ eluting coronary bifurcation stent system is a conically shaped self-expanding DES, constructed from a nickel-titanium alloy (nitinol). The stent is designed to dynamically adapt to the Y shape of the carina up to an angle of 70o, maintaining native geometry without metallic struts, and facilitating long-term SB access (Figure 2).
The stent has an abluminal polymer coating composed of polylactic acid, that is biodegradable into carbon dioxide and water within 6–9 months of deployment, and the semi-synthetic sirolimus analogue Biolimus A9™, which has antiproliferative effects and has been proven superior over first-generation sirolimus-eluting DES.9 The AXXESS™ stent is CE marked and has UK approval. It is currently available in 3.0 mm (expandable to 3.75 mm) and 3.5 mm (expandable to 4.25 mm) diameters, and in two lengths (11 and 14 mm). A 4.0 x 9 mm device has been developed and used in a limited number of cases in the UK, mainly in the left main stem (LMS), where a bifurcation angle of 120˚ can be spanned. However, this iteration of the device it is not currently available.
The AXXESS system is conventionally 7 French (7 Fr) compatible, although can be delivered through a specific 6 Fr guide catheter with an internal lumen diameter of 0.072" (e.g the Adroit guide, Cordis Miami, Florida, USA; but only with a single guide wire). It is delivered through a hydrophilic-coated rapid-exchange system comprising a delivery catheter, mounted stent and cover sheath (Figure 2). The stent is delivered following gradual withdrawal of this sheath, with the technique for implantation being described in detail below. One radiopaque gold marker exists at the proximal edge, with three at the distal edge arranged at equal intervals on the circumference to visually guide optimal placement (Figure 2). It is positioned spanning the carina into the ostium of both the MV and the SB.
Indications for Implantation
Any Medina classification of bifurcation can be treated using the AXXESS system. However, the angle between the MV and SB should not be greater than 70o and, ideally, the SB should be a minimum of 2.5 mm in diameter and have minimal calcification. In order to clearly define the bifurcation angle, two orthogonal radiographic projections are often required. As it elutes Biolimus A9, the standard contraindications for drug-eluting devices apply. There are no restrictions in location, with devices being used successfully throughout the coronary circulation, including the left main bifurcation;10,11 however, it cannot be overexpanded and careful vessel sizing (often requiring intravascular imaging) should be performed. It can be implanted for the full range of coronary interventional indications, including acute coronary syndromes and stable angina.12
AXXESS is particularly useful in the treatment of Medina class 1,0,0 lesions, as it is often the only device required in these cases. When treating all other Medina classes, additional distal overlapping DES in either MV or SB may be required.
Technique of Implanting an AXXESS™ Stent
Although the AXXESS device can be delivered through any 7 Fr-guiding system, when considering guide shape, support should be maximised to facilitate device delivery. Two separate guide wires (any moderate support 0.014" wire) should be advanced distally into the MV and SB. The use of dedicated stents, particularly self-expanding devices, require thorough lesion preparation, particularly in more complex calcified lesions (e.g. Medina 1,1,1). Consequently, noncompliant balloons, cutting balloons and adjunctive devices such as rotational atherectomy should be considered early to optimise final position, minimal luminal area and stent expansion. Passage of a ‘winged’ cutting balloon distal to the target lesion is an indicator that the AXXESS device can be delivered into the appropriate position. Predilatation is therefore strongly recommended before delivering the AXXESS stent, with vessel preparation of both MV and, in the majority of cases, into the ostium of the SB if disease is present.
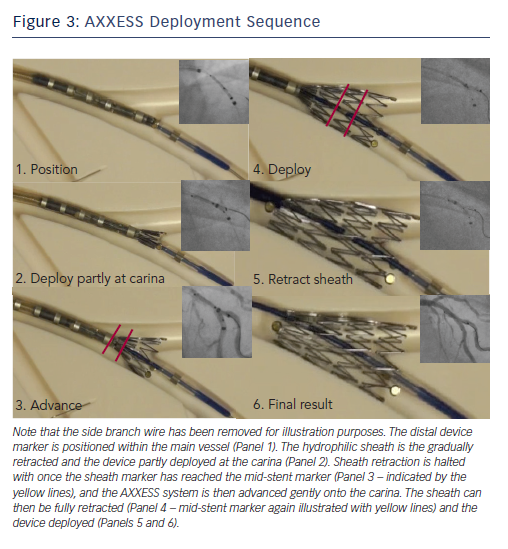
The AXXESS™ stent is advanced onto the wire of the distal vessel with the sharpest bend to the proximal MV. This can be either branch, but in a series of over 100 implanted devices evaluated by our group it was positioned on the MV wire in 92 % of cases.10 The distal markers of the AXXESS stent are advanced beyond the carina in the distal vessel. The whole device is then gently retracted, alongside simultaneous gradual pullback of the actuator, slowly retracting the sheath. This allows progressive flaring of the three distal markers across the carina. Angiographically, this is visualised when one marker appears to be in either MV or SB, with the other two markers positioned in the other vessel, spanning the carina. While the cover sheath contains more than half the stent length (identified by the deployment marker on the delivery catheter), further adjustment of the stent position is possible. It is often helpful to confirm adequate carina spanning by gently advancing the device forward while the cover sheath remains in this position, as adjustments can still be made at this point. This should be visualised in two orthogonal radiographic projections to ensure separation of the three markers into the MV and SB. It should be stressed, however, that the device cannot be recaptured and the cover sheath cannot be re-advanced over the expanded stent.
The final positioning manoeuvre occurs with gentle forward pressure applied with the device spanning the carina, and full retraction of the covered sheath using the actuator. Ideally, the device should be placed 2–3 mm distal to the carina to maximise distal vessel stent coverage (Figure 3).
Following deployment of the AXXESS stent, the jailed wire can then be withdrawn and reintroduced if needed, with easy access maintained into the other branch. Further stents can then be advanced if required and deployed in the distal MV or SB. If a distal stent is required, then the operator should aim for a minimal overlap of 1–2 mm between the AXXESS and any further stents. Similarly, significant proximal disease to the edge of the AXXESS stent in the MV can be treated with overlapping DES as appropriate. In the authors’ experience, Biomatrix devices have been used, conventionally, as they carry the same polymer and drug, although any DES could be used. As the AXXESS stent is self-expanding, post-dilatation is often not required. If no stent is placed in the SB distal to the AXXESS stent, often a final hugging balloon dilatation with the SB is performed. An appropriate non compliant (NC) balloon is placed within the distal marker of the AXXESS stent in the SB against an NC balloon in the MV. If both MV and SB were stented distal to the AXXESS stent, then standard final kissing balloon dilatation (FKBD) should be performed in the majority of cases.
Tips and Tricks
The successful deployment of an AXXESS device is achievable by the majority of PCI operators; however, the simple steps outlined above should be followed to minimise this risk of complications. It is advisable to undergo a short period of proctoring before attempting implantation solo as the delivery of the device differs from a conventional balloon expandable stent, as is detailed above. Adjunctive use of intracoronary imaging permits clear definition of the bifurcation anatomy and suitability for a dedicated device, and also facilitates appropriate lesion preparation that permits optimisation of the final stent result. It is recommended in the early learning curve of a new operator that such imaging is performed. While AXXESS can be used safely in the left main stem, it is not advisable for AXXESS implantation to be attempted in this circumstance until a number of successful AXXESS deployments have been undertaken in non-left main bifurcation lesions.
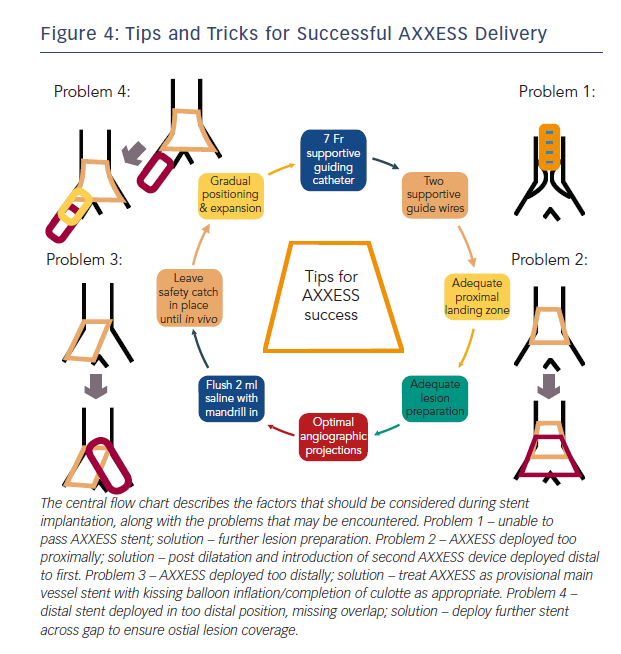
There are a small number of potential problems that maybe encountered during implant that can be mitigated by careful technique. These are outlined below, along with simple solutions that are effective in the majority of cases.
Problem 1: Failed to deliver AXXESS
Delivery device failure should be considered a possibility when planning the case. However, with a careful strategy and appropriate equipment this risk is minimised. As detailed above, it is important to select a supportive guiding catheter along with good distal supportive guide-wires at the outset. Lesion preparation is crucial, with ideally 1:1 balloon sizing and predilation with NC, with or without cutting balloons as appropriate. Failure to prepare the vessel adequately is the most common reason for device delivery failure and can easily be overcome with more debulking. It is safe to remove the device if the cover sheath remains fully closed but caution should be taken once the distal stent is exposed. If this circumstance is encountered, it is safer to deploy in the main vessel rather than trying to remove the device once cover sheath retraction has commenced.
Problem 2: AXXESS Delivered too Proximally
Device being delivered too proximally can be a consequence of problem 1 or hasty device deployment. If this has occurred, with the struts clearly proximal to the carina and not spanning the ostium of either branch, then (after appropriate post dilatation as required) a second AXXESS device may be delivered through the first and placed in a more appropriate position distally. Care should be taken to remove the SB wire prior to AXXESS deployment to avoid wire trapping.
Problem 3: AXXESS Delivered too Distally
If the device is delivered too distally, it will be unable to expand fully and span across the carina. This may be corrected if the device is only just distal of the carina with a kissing balloon inflation that may flare the struts across the carina. If this is ineffective, or the device is positioned in such a way that this is clearly not possible, then it can be used safely and effectively as a provisional MV stent. The SB may be accessed through the device and the procedure completed using a culotte technique with FKBD if SB stenting is required or a provisional approach can be adopted.
Problem 4: Distal Stent Overlap
If a stent is required distally to the AXXESS device, then care should be taken with the positioning of the proximal stent edge to ensure overlap. If there is a suspicion that a gap remains between the two devices, then this should be confirmed using intracoronary imaging (intravascular ultrasound [IVUS] or optical coherence tomography [OCT]) and a further short stent used to cover as appropriate.
The majority of problems with the delivery and positioning of AXXESS can be predicted. By simply considering the factors detailed in Figure 4 when planning the procedure, the risk of a serious complication can be minimised.
Outcomes
There is a growing body of literature that supports the use of the AXXESS system in the treatment of coronary bifurcation lesions. The first-in-man AXXESS PLUS trial reported results at 6 months in 139 patients who underwent implantation across 13 centres, with low rates of TLR (7.5 %) and late-lumen loss (0.09 mm).13 There was a low rate of periprocedural complications (MACE rate 5% (n=7), non-Qwave MI 4.3% (n=6)), with a late-stent thrombosis rate of 2.1 % (three patients, two of who associated with premature cessation of antiplatelet therapy).
This trial has been followed with the prospective DIVERGE study, that assessed 302 patients across 14 centres in Europe, Australia and New Zealand.14 The cumulative MACE rate at 9 months was 7.7 % (0.7 % death, 3.3 % non Q-wave MI, 1.0 % Q-wave MI and 4.3 % TLR). This is comparable to the equivalent MV stent (Biomatrix) with the 9-month MACE rate in the Biolimus arm of the LEADERS trial being reported as 9.2 %.15 Importantly, 64 % of patients treated with AXXESS had additional DES (in both MV or SB) and all DES used were first-generation sirolimus-eluting devices. Stent thrombosis was observed in 1.0 % of patients (0.7 % subacute and 0.3 % late), the overwhelming majority of which occurred within the additional DES rather than the AXXESS device. The overall restenosis rate was 6.4 %. Both the AXXESS PLUS and the DIVERGE study have now reported 5 years results – pooled data represent a patient population of 432 patients. The overall MACE rate was reported at 21.3 % (6.5 % death, 8.6 % MI and 12.4 % ischaemia-driven TLR).16 This is comparable to the MACE rate of 22.8 % observed in the Biolimus arm of the LEADERS study at 5 years,9 and to the 5-year MACE rate of at 18.3 % in the MV-only arm in the NORDIC bifurcation trial.3
Importantly, among the DIVERGE cohort, 36 % of patients with true bifurcation lesions were treated using a single device only, or with a single additional stent in either MV or SB.17 This is an equivalent approach to a provisional technique, but maintaining SB access, and leaving the carina free from metallic scaffold. Indeed, the MACE rate in this sub-group was 18 %, again comparable to the provisional MV-only arm of the NORDIC trial.3 Importantly, this was not significantly different to the MV-plus-SB group using AXXESS (MACE rate of 23.3 %). Whereas, when a two-stent technique was employed within the NORDIC trial the overall MACE rate was 28.2 %, which was significantly different to the MV-only stent arm. The overall MACE rate for the MV arm of nordic bifurcation at 5 years was 18.3 %.
The DIVERGE14 and AXXESS PLUS trial13,15 both had stringent inclusion criteria, with the treated lesion dimensions restricted to just 10 mm extension into the MV proximal to the bifurcation, and 15 mm into either branch (MV and SB). They excluded lesions with angiographically apparent heavy calcification. It may be considered that this does not reflect ‘real-world’ practice, but observational data are available to support the use of AXXESS in more complex lesion subsets. In our data series from over 100 implants across four UK centres, the device has been successfully deployed in a variety of situations, including heavily calcified vessels that require rotablation, chronic total occlusions and in vessels that contain multiple bifurcations and where angles exceed 70˚. The overall complication rate was low, with a single access site-related retroperitoneal bleed, and a single coronary perforation following post dilatation of the MV AXXESS stent, representing a 2 % complication rate,12 similar to that in other published series.16
The single randomised trial in the literature, the COBRA study,18 compares the use of the AXXESS system alongside Biomatrix stents with a two-stent culotte strategy using Xience Pro stents (Abbott Vascular, Illinois, USA) in a cohort of 40 patients with true bifurcation lesions. The trial was conducted at two European centres, with FKB inflations being performed in all cases. All patients were restudied at 9 months with quantitative coronary angiography (QCA) and OCT. There were no significant differences observed in the OCT primary endpoint – the proportion of uncovered struts per bifurcation segment – with a trend towards a higher proportion of uncovered struts in the AXXESS group. However, both the mean stent area and mean lumen area in the proximal MV were significantly greater when the AXXESS system had been used. This was consistent with the QCA findings, with in-stent late-lumen loss significantly lower within the AXXESS arm at both the bifurcation core and within the segment of disease covered with the AXXESS stent (0.04 mm versus 0.39 mm; p=0.002) There was no significant differences in clinical outcomes reported at 1 year, as could be expected from this small cohort.
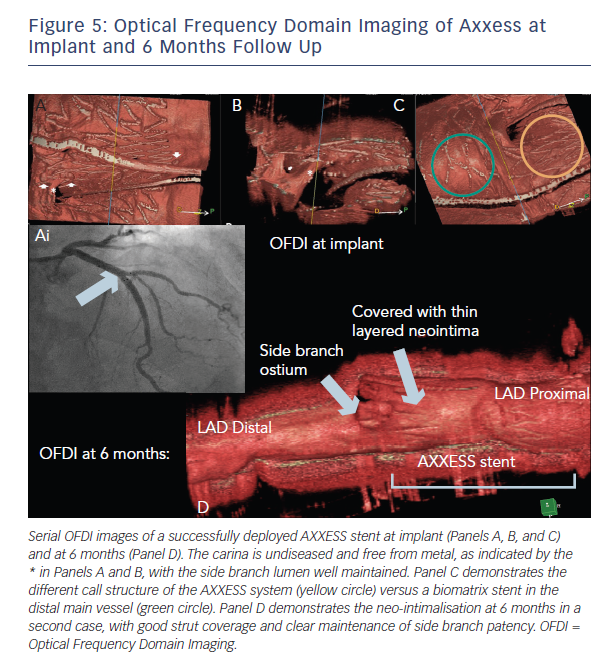
This finding is consistent with the results of the IVUS subgroup of the DIVERGE study that demonstrated a 26 % increase in luminal volume at 6 months.19 This is likely to be a consequence of the self-expanding nature of the AXXESS device, maximising luminal gain from a PCI bifurcation procedure. The OCT findings at 6 months are illustrated in Figure 5.
There remains a lack of large-scale prospective randomised data evaluating the use of the AXXESS system. Despite this, cohort reports and the prospective data presented above have indicated that the system is safe, effective and with rates of adverse outcomes that are comparable to to that observed in large-scale randomised biolimus-eluting stent trials.
Conclusion
The AXXESS system presents a novel solution for the treatment of de novo coronary bifurcation lesions. It has been proven to be associated with low rates of MACE and stent thrombosis, and has a number of theoretical benefits over conventional two-stent strategies. Prospective data support its use in complex coronary bifurcations, however, its use has yet to be studied in a large-scale randomised controlled trial.