Artificial intelligence (AI) is a broad term that implies the use of machines to mimic human behaviour and perform various actions with minimal human intervention.1,2 Machine learning (ML), a branch of AI, can analyse information and discover hidden patterns in data.3
The treatment of cardiovascular disease has significantly evolved in interventional cardiology over the last 2 decades.4 Although percutaneous coronary intervention (PCI) is the cornerstone of the catherisation laboratory, many conditions can be treated there, including coronary artery disease (CAD), valvular heart disease, cardiac arrhythmias, pericardial disease, myocardial disease, congenital heart disease and heart failure.
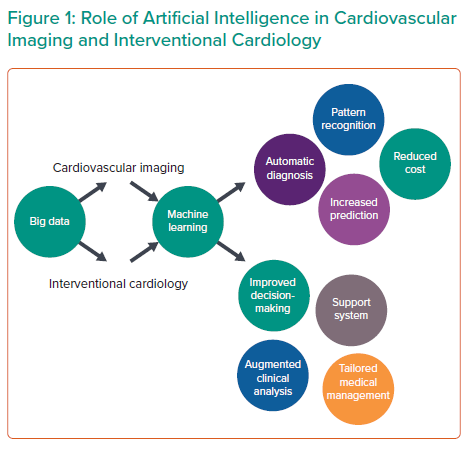
With the emergence of transcatheter therapies, the clinical arena of interventional cardiology has greatly expanded. Non-invasive imaging is the critical gatekeeper in the assessment of cardiovascular diseases before cardiovascular intervention.5 AI technologies in imaging are demonstrating their capacity for image interpretation, quality control, diagnostics and improved workflow.6 AI and ML can help discover new variants or phenotypes present within large data in cardiovascular imaging, which can improve our understanding and lead to new therapeutic interventions in CAD.7 They can further aid in interventional cardiology as they can improve clinical decision-making, organise workflow in the catherisation laboratory, facilitate catheter-based intervention through robotic application and predict proper placement to reduce or avoid paravalvular leakage.4,8
Over the last few years, AI has substantially altered the landscape in clinical medicine by providing new insights and opportunities to improve therapy.6 While AI is evolving in other aspects of human life from self-driving cars to automated voice recognition systems, ML is expanding clinical pathways and opening new frontiers in cardiovascular medicine.9–14 In comparison, the application of ML in interventional cardiology (IC) has been less apparent.13,14 It is evident that AI progress in IC lags behind its counterparts but interest in it is still growing.14 As advances in stent technology and transcatheter aortic valve replacement (TAVR) or transcatheter mitral valve replacement (TMVR) continue, AI will be beneficial.6
In this review, we will discuss how AI will improve the role of cardiovascular imaging and imaging in interventional cardiology.
Potential of Artificial Intelligence in Interventional Cardiology
AI has tremendous capabilities with transformative potential and can perform a wide variety of functions.6 These include pattern recognition, problem-solving, identification of objects and sounds and language comprehension.15,16 In the simplest terms from a clinical standpoint, AI can make data-driven decisions to evaluate disease progression or select the most appropriate treatment.17
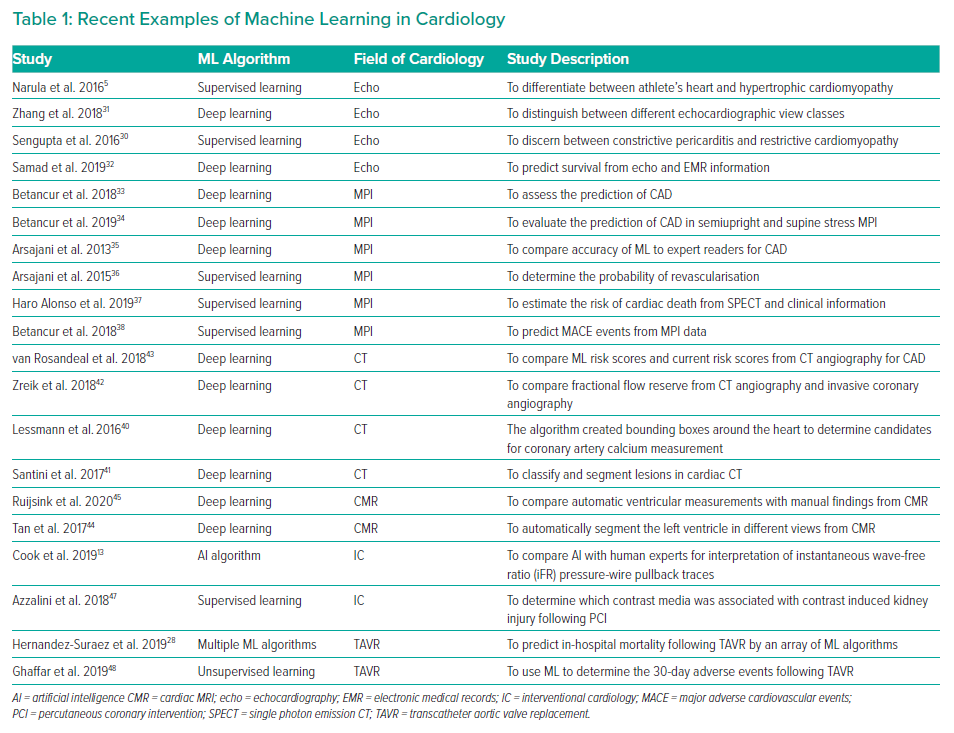
Although AI has produced substantial findings in cardiovascular imaging and electrophysiology (Table 1), the role of AI in IC is still in its infancy (Figure 1).4,12 Currently, the practice of AI in IC can be broadly divided into two major disciplines: virtual and physical.14 The virtual branch includes ML algorithms, natural language processing (NLP), cognitive computing and automated clinical decision support systems, whereas the physical branch is mainly restricted to robotic interventional procedures.
Types of Machine Learning
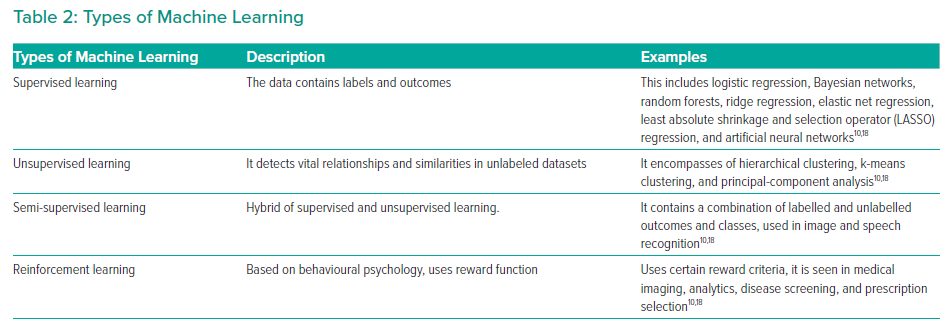
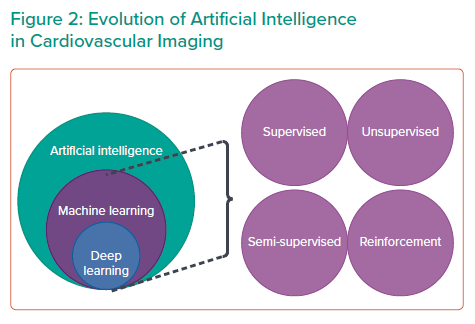
ML is a collective term that encompasses a variety of algorithms (Table 2). This includes supervised learning, unsupervised learning and reinforcement learning. The choice of algorithm depends on the indication and purpose of the investigation. Supervised learning uses specific labels or classes.18 Unsupervised learning analyses a database without labels.10 Semi-supervised learning is a combination of supervised and unsupervised learning.19 Reinforcement learning uses reward criteria similar to human psychology to perform actions.18 Deep learning is one of the most advanced ML algorithms available.20
Supervised and unsupervised learning have demonstrated tremendous potential in cardiovascular imaging, and deep learning (DL) is poised to make the evolutionary leap in ML for cardiovascular imaging.2 It does not require significant training to execute an action.7 It performs significantly better with larger and more complex data sets than other ML algorithms (Figure 2). It requires less domain knowledge to perform various tasks. Among the various algorithms available, DL has the most potential and prowess for prediction.9,11 It is being used in a variety of applications, including voice recognition systems, such as Amazon’s Alexa or Apple’s Siri, and image identification systems.10 DL may process complex information through several layers to process information. It is extracted through each part of the hierarchy and DL can recognise various hidden patterns.9 DL has tremendous capabilities, which are being used in various sectors by the commercial industry and information technology.6
Natural Language Processing
NLP is a branch of AI that comprehends the mechanics of human language.14 This method can be applied to electronic medical records (EMRs) for large-scale text analysis and data extraction. Similar to ML algorithms, it may be used to identify complications and postoperative events to streamline clinical workflows.21,22 Furthermore, NLP can be used to analyse data from heterogeneous sources with various formats, which can be advantageous to IC. It has been applied in risk scoring, adverse drug event identification or patient selection for cardiac resynchronisation therapy, to name a few.23–25
Computer Vision
Computer vision uses image processing and ML algorithms to detect features or pattern recognition in digital or video imaging.18,26 Computer vision algorithms coupled with deep learning can provide an automated interpretation of complex radiological images to assist or train cardiologists.27 This may play an important role in IC for identifying potential complications in the placement of valves and structural defects.14,28 Currently, there are troves of IC data that can be analysed by ML algorithms to predict adverse events.
Clinical Decision Support System
Clinical decision support systems use information in EMRs and present summaries and notification that are relevant to healthcare providers in implementing evidence-based clinical practice. They may provide information regarding risk factors, drug interaction information or information relevant to patients undergoing catheterisation. Because it relies on EMRs, NLP could be imperative to decision support systems. However, ML could also be used to interpret images for treatment approaches and aid in medical management.14
Role of AI in Echocardiography
Echocardiography is the primary image modality in cardiology and plays a central role in most diagnostic pathways for several pathological entities.19 In several recent studies, ML algorithms have shown innovative applications in findings using echocardiographic parameters.11 Although echocardiography is widely used, its results can be heterogeneous.29 These variables can affect management and outcomes for the interventionist. Machine learning can produce rapid and consistent findings, which can help budding interventionists in clinical decision-making.29
In our own experience, we have harnessed the capabilities of AI to identify differences between hypertrophic cardiomyopathy and athlete’s heart, and applied a supervised learning algorithm to differentiate between restrictive cardiomyopathy and constrictive pericarditis.5,30
Similarly, Zhang et al. used a convolutional neural network (CNN), a DL algorithm, for automated image interpretation.31 The ML algorithm was trained to identify 23 viewpoints and segmentation of cardiac chamber across five common views. Impressively, the algorithm was successful in identifying views (96% for parasternal long axis) and enabled the segmentation of cardiac chambers.
Samad et al. used a random forest ML algorithm to predict survival by using echocardiography measurements and electronic medical information in 171,510 patients.32 The random forest models demonstrated superior prediction accuracy (all AUC [area under the curve] >0.82) over common clinical risk scores (AUC 0.69–0.79). Also, the ML models outperformed logistic regression models (p<0.001 and all survival durations).
Role of AI in Nuclear Medicine
Myocardial perfusion imaging (MPI) has an important role in nuclear medicine and provides vital information in CAD.9 Single-photon emission CT (SPECT) enables physicians to identify perfusion defects. MPI is a non-invasive modality that has a paramount role in risk stratification for CAD.9 With ML algorithms, it can integrate clinical information and a large number of parameters to predict CAD, revascularisation and major adverse cardiovascular events (MACE).16 These aspects can be particularly useful for interventionists as they can assist in clinical management and patient selection for high-risk procedures.
Betancur et al. assessed the prediction of obstructive coronary artery disease (CAD) using myocardial perfusion imaging (MPI) by a DL algorithm.33 The ML algorithm demonstrated a higher area under the receiver-operating curve than total perfusion deficit (TPD) for CAD prediction (per patient 0.80 versus 0.78; per vessel 0.76 versus 0.73; p<0.01). Recently, Betancur et al. also assessed a deep learning algorithm for the prediction of obstructive CAD with a combination of semi-upright and supine stress MPI in comparison with TPD.34 Similarly, the area under the receiver-operating curve for prediction of disease per patient and per vessel by the ML algorithm was better (per patient, 0.81 versus 0.78; per vessel 0.77 versus 0.73, p<0.001).34 Arsajani evaluated the combination of clinical and imaging data to predict CAD using SPECT.35 The receiver-operating curve for the ML method was better than TPD and two readers with considerable significance (p<0.001).
Arsajani et al. examined clinical and imaging data to determine the probability of revascularisation in 713 patients with suspected CAD through a LogitBoost supervised learning algorithm.36 The specificity of the ML algorithm was better than both expert readers (p<0.05) and similar to total perfusion deficit (p<0.05). In addition, the receiver-operating curve for the ML architecture (0.81 ± 0.02) was similar to reader 1 (0.81 ± 0.02), but better than reader 2 (0.72 ± 0.02; p<0.01) and the standalone measure of perfusion (0.77 ± 0.02; p<0.01).
Alonso et al. used a supervised ML algorithm to estimate the risk of cardiac death, derived from an amalgamation of adenosine myocardial perfusion SPECT with clinical information in 8,321 patients and 551 cases of cardiac death.37 The logistic regression was outperformed by the ML algorithm (AUC 0.76; 14 features). It evidently showed superior accuracy (AUC 0.83; p<0.0001; 49 features). Nonetheless, the least absolute shrinkage and selection operator (LASSO) model required the least number of features (AUC 0.77; p=0.045; six features).
Betancur et al. explored the predictive value of patient information with SPECT MPI to predict MACE through a LogitBoost supervised learning ML algorithm in 2,619 patients.38 At around 3 years’ follow-up, 239 patients had experienced MACE. Interestingly, the ML combined achieved superior MACE prediction than ML imaging (AUC 0.81 versus 0.78; p<0.01). The ML also had higher MACE predictive accuracy when compared with an expert reader, automated stress total perfusion deficit and automated ischaemic perfusion deficit (AUC 0.81 versus 0.65 versus 0.73 versus 0.71; p<0.01 for all).
Role of AI in Cardiac CT
CT is a non-invasive approach for the identification of obstructive CAD. CT enables the depiction of the underlying coronary anatomy to visualise plaques or stenosis in the coronary artery tree.39 From an interventionist point of view, cardiac CT plays an important role in appropriate selection for PCI. Among all non-invasive modalities, CT angiography closely mirrors invasive angiography. ML algorithms can greatly augment the possibilities of cardiac CT.
Lessmann et al. used a convolutional neural network (CNN), a deep learning algorithm, to create a bounding box around the heart that corresponds to certain Hounsfield units.40 They investigated the potential of an automated coronary calcium system that was able/would be to screen patients for high-risk cardiovascular events. Santini et al. explored the role of a CNN algorithm in classifying and segmenting lesions in cardiac CT imaging.41 After proper training of the CNN algorithm with various CT volumes, they were able to demonstrate a Pearson correlation measuring 0.983.
Zreik et al. used a CNN algorithm to automatically calculate the fractional flow reserve (FFR) from coronary CT angiography.42 Surprisingly, there was good agreement between the ML-derived values and invasively measured one, with the AUC being 0.74. Rosendael et al. examined the role of a boost ensemble algorithm to compare ML-derived scores and current risk scores in CT angiography for CAD evaluation.43 The events expressed by the AUC was superior by the ML algorithm in reference to conventional scores (0.771 versus 0.685 to 0.701; p<0.001).
Role of AI in Cardiac MRI
Cardiovascular magnetic resonance (CMR) imaging has emerged as a robust diagnostic modality for assessing a variety of clinical conditions in cardiology. Of the various non-invasive approaches, it is the only option that permits tissue characterisation. CMR can be used by the interventionist to plan appropriate management for patients. During procedures, CMR imaging can be used to guide complex procedures because of the minimal risk of radiation.
Tan et al. assessed the role of the CNN algorithm for automatic segmentation of the left ventricle in short-axis slices in publicly available database.44 Interestingly, the ML algorithm achieved a Jaccard index of 0.77 for the left ventricle segmentation challenge dataset and obtained a continuously ranked probability score of 0.0124 for the Kaggle Second Annual Data Science Bowl.
Similarly, Ruijinsk et al. evaluated the role of the CNN framework for automated ventricular function assessment from cardiac CMR.45 The findings corroborated highly with manual analysis for left ventricular and right ventricular volumes (all r >0.95), strain (circumferential r = 0.89; longitudinal r >0.89) and ejection rates (all r ≥0.93).
Can AI-integrated Cardiovascular Imaging Facilitate Interventional Therapies?
Using the vast troves of cardiovascular imaging enables the possibilities of data-driven phenotypic differentiation.46 This has particular relevance in the field of IC and transcatheter therapies for enabling individualised therapies.5 Algorithms integrating ML and cardiovascular imaging can help generate patient-specific risk scores, which can yield diagnostic significance in procedural planning.14 Furthermore, ML may play a paramount role in automating cardiovascular imaging workflow for referral of patients to cardiac catherisation laboratory by facilitating faster reading, interpretation and diagnosis.5,14 In addition, ML algorithms may help predict outcomes such as mortality or complications following interventional therapies.
Role of AI in Percutaneous Coronary Intervention and Transcatheter Aortic Valve Replacement
Although the potential of AI has not been fully harnessed in PCI, a few studies provide a glimpse of AI in the near future.14 One aspect of considerable variability in interpretation is physiologically guided coronary revascularisation.8 The consistency of results can be improved with AI.
Cook et al. compared an AI algorithm with 15 human experts for interpretation of 1,008 instantaneous wave-free ratio (iFR) pressure-wire pullback traces.13 In addition, a heart team interpretation was determined by a consensus of individual opinions. The median human expert had an 89.3% agreement with heart team response, while it was 89.4% with AI (p<0.01 for non-inferiority) for PCI haemodynamic appropriateness. Within the 372 cases evaluated for haemodynamic appropriateness, the AI framework had 89.7% agreement while the median human expert was 88.8% in agreement with the heart team response (p<0.01 for non-inferiority). Cook et al. confirmed that the AI algorithm was not inferior to the expert decision making for determining the appropriateness and strategy of PCI.13
Azzalini et al. used a generalised boost regression to determine which contrast media among five types was associated with contrast-induced kidney injury following PCI in 2,648 patients.47 In a risk-adjusted analysis, Azzalini found no particular contrast type was associated with contrast-induced kidney injury when compared to iodixanol.
Currently, few centres have explored the role of AI in TAVR. Hernandez-Suarez used ML algorithms to predict in-hospital mortality after TAVR in 10,883 patients using data derived from the national inpatient sample.28 The AUC for the ML algorithm was greater than 0.80. They were able to show ML could generate risk models capable of predicting in-hospital mortality.
Ghaffar explored the role of topological data analysis (TDA) network to predict 30-day complications and mortality following TAVR in 228 patients.48 Four clusters were identified. Cluster A had more frequent vascular intervention (p<0.016), while clusters B and D had a higher number of procedures before TAVR (p<0.04). Clucert C underwent TAVR. Major adverse complications were seen in the first week of complications in clusters C and D (p<0.05). Interestingly, there was no difference in the Society of Thoracic Surgeon’s risk scores between either cluster.
Role of AI in Robotics
Interest is surging in robotic technology for IC because it can deliver precise, efficient clinical care.4 Robotic technology has the capability to reduce variability in procedure time, accurately assess lesion length and decrease the number of stents used.4 Robots can be used in training young interventionists.
Though robotics has the potential to open new doors in IC, it is not without flaws.14 There are fundamental differences between interventionists and their accompanying robotic assistants. These systems do not recognise the underlying anatomy or understand the intentions of the operator. ML algorithms, computer vision and image interpretation can truly help in this process by bridging the gap between man and machine.26 They can enhance the underlying technology to possibly enable some degree of automation and possible decision-making.14 Future robotic systems may be able to assess previous procedures and provide feedback to interventional cardiologists.
Our Evolving Views on AI in Cardiovascular Imaging and Interventional Cardiology
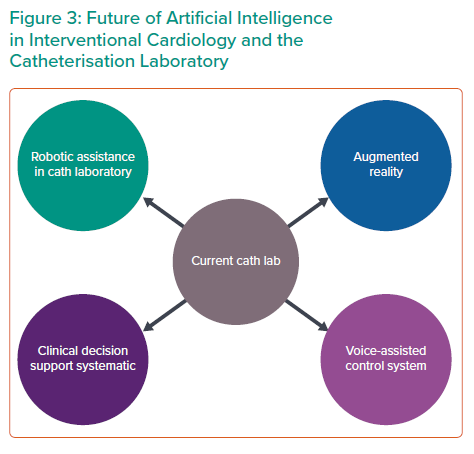
Although AI has clearly caused paradigm shifts in cardiovascular imaging, the application of AI for IC is still in its early stages.14 AI is poised to create revolutionary progress in IC in years to come (Figure 3). AI offers the possibility of detecting patients with high-risk profiles and gauge treatment effects according to various factors. In the catherisation laboratory, AI can assist in procedural guidance for angiography, intravascular imaging and provide any form of additional support to the operator during the procedure.4
Shortly, AI could provide patient-specific, vessel-specific or even lesion-specific revascularisation strategies.8 Based on outcome data arising from national and international registries, this could be tapped by AI to create treatment strategies to improve short- and long-term outcomes.
Cardiologists must be cognisant of the risks of AI and be educated on its strengths and weakness.20 They must not blindly accept the actions of AI. By being aware of the risks, we can fully tap the potential of AI in IC.
Many echocardiographers have played a key role in the development of AI and ML algorithms in cardiovascular imaging.12 Similarly, interventionists must play an active role in the genesis and propagation of AI in IC.8 Interventionists can provide clinically relevant information to engineers regarding the nature of IC data. Engineers can use this data to create practical solutions in the field of IC. Such algorithms could benefit the interventionist, streamline the workflow and help to minimise error. Collaboration between interventionists and engineers needs to occur at national and international levels for AI to truly flourish in IC.
Conclusion
AI is playing a paramount role in diagnostic imaging by integrating vast amounts of information. Similarly, AI is beginning to take root in IC. It can expand options for procedural guidance, intraprocedural analysis, robotics and clinical judgement.
As interventionists learn to adopt AI in clinical practice, it will revolutionise IC treatment strategies. There may be some initial hurdles, and difficulties are inevitable in the path to progress. The trinity of human, machine and patient will be the focal point of IC along with imaging in years to come.