Stent under-expansion carries a major risk of stent thrombosis with serious consequences if left untreated. There have been reports of using rotational atherectomy (ROTA) to overcome stent under-expansion, but the procedure holds many risks. Intravascular lithotripsy (IVL) can only be carried out if the balloon passes through the lesion. If scarring or fibrotic tissue is present, the chance of successful IVL treatment decreases. Excimer laser coronary angioplasty (ELCA) could be used on a regular guidewire and the fibres emit ultraviolet light pulses from the front surface, making photoablation effective even if the minimum stent area (MSA) is very small. If combined with super high-pressure angioplasty balloon (OPN non-compliant [NC] balloon), the chance of successful percutaneous coronary intervention (PCI) is much higher.
Case Example
The patient was referred to the Department of Cardiology, University Medical Center, Szeged, with recurrent angina for repeat coronary angiography and a Canadian Cardiovascular Society (CCS) score of III. The patient’s invasive cardiology medical history dated back 18 years, when the circumflex artery (CX) and the right coronary artery (RCA) were treated with bare metal stents due to non-ST elevation myocardial infarction. According to the description, full stent expansion could not be achieved in the RCA. Several years later, the left anterior descending artery (LAD) was treated with a drug-eluting stent (DES) and another stent was placed with difficulties in the prior treated RCA proximal segment. Within hours after PCI, due to intensifying chest pain and ST elevation in inferior leads on ECG, urgent coronary angiography was performed. RCA proximal stent occlusion was discovered. The under-expanded double stent layer segment was treated with ROTA with off-label indication with a 1.5 mm burr (Boston Scientific), but burr retrieval was difficult. Dual antiplatelet therapy (aspirin and clopidogrel because a more potent drug was not available at the time) was carried out.
Due to progressing symptoms several years later, a nuclear stress test was performed, which demonstrated inducible ischaemia in the inferior lateral wall segments. Echocardiography revealed an EF of 45% and multiple inferior lateral wall segmental hypokinesia. Mitral regurgitation was grade II–III, tricuspid regurgitation was grade II.
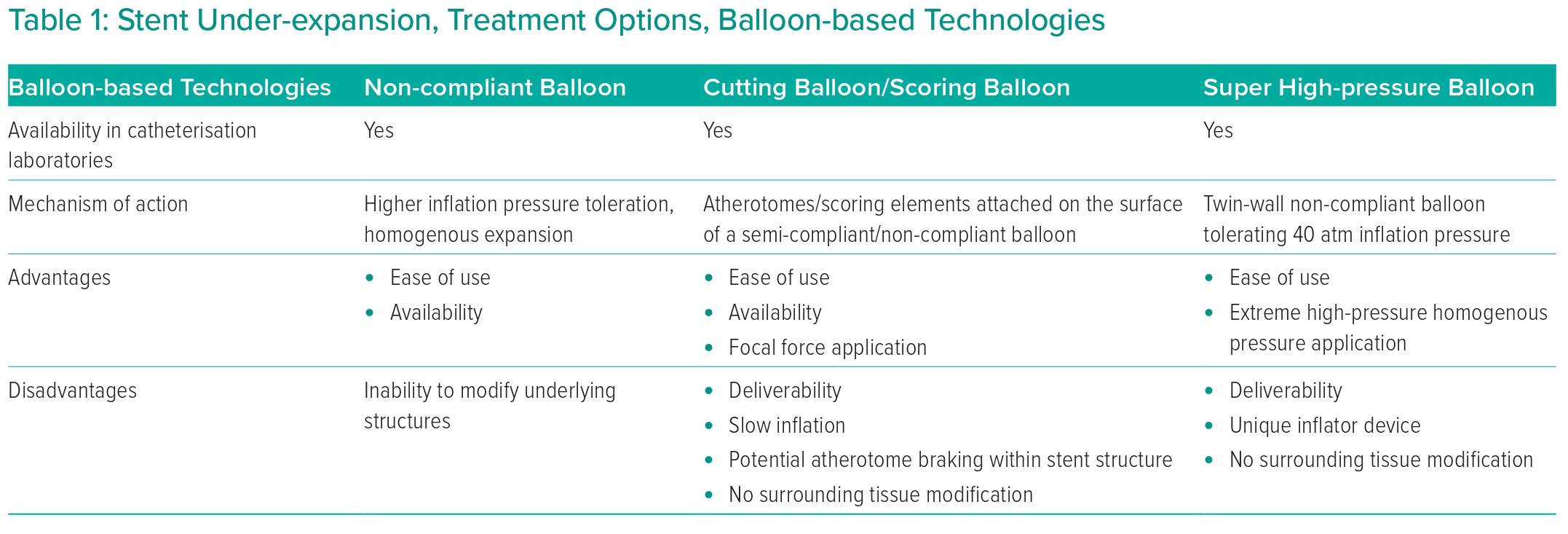
Coronary angiography revealed patent LAD stent, a small-diameter diffusely diseased CX with no restenosis or significant lesion (no information on previous stent sizes). A 20% stenosis of the RCA ostium, a very tight calcified lesion in the proximal RCA segment (stent material visible) and significant lesions at the crux (Figures 1A and 1B) were discovered. The distal RCA showed only mild lesions (Figure 1A).
The decision was to perform PCI using laser atherectomy. Optical coherence tomography (OCT) was carried out pre-PCI to assess RCA lesions intended to treat (Supplementary Material Video 1). At the site of the proximal tight calcified stented segment, the smallest measured minimum stent area (MSA) was 1.96 mm2. Calcification was prominent, particularly at 3 o’clock (Figure 1A [Aa and Ab OCT images]), but no shadows of stent struts were present at this location. At 9 o’clock multiple concentric layers of stent struts were spotted with shadows obscuring the vessel wall, indicating that there were crushed stent segments (Figure 1A, [Aa and Ab OCT images]). The distal mid-RCA segment showed a moderately calcified significant lesion, with the take-off of the acute marginal branch. The MLA was 2.26 mm2 (Figure 1A [Ac OCT image]).
Non-compliant balloon inflation attempt (4.5 × 15 mm) at 20 atm (Figure 1C) was carried out at the site of the under-expanded stented segment, but angiography (Figure 1C) and OCT (Figure 1C [Ca OCT image] and Supplementary Material Video 2) showed only a minimal increase in the MSA (2.8 mm2) with torn intima parts in the lumen.
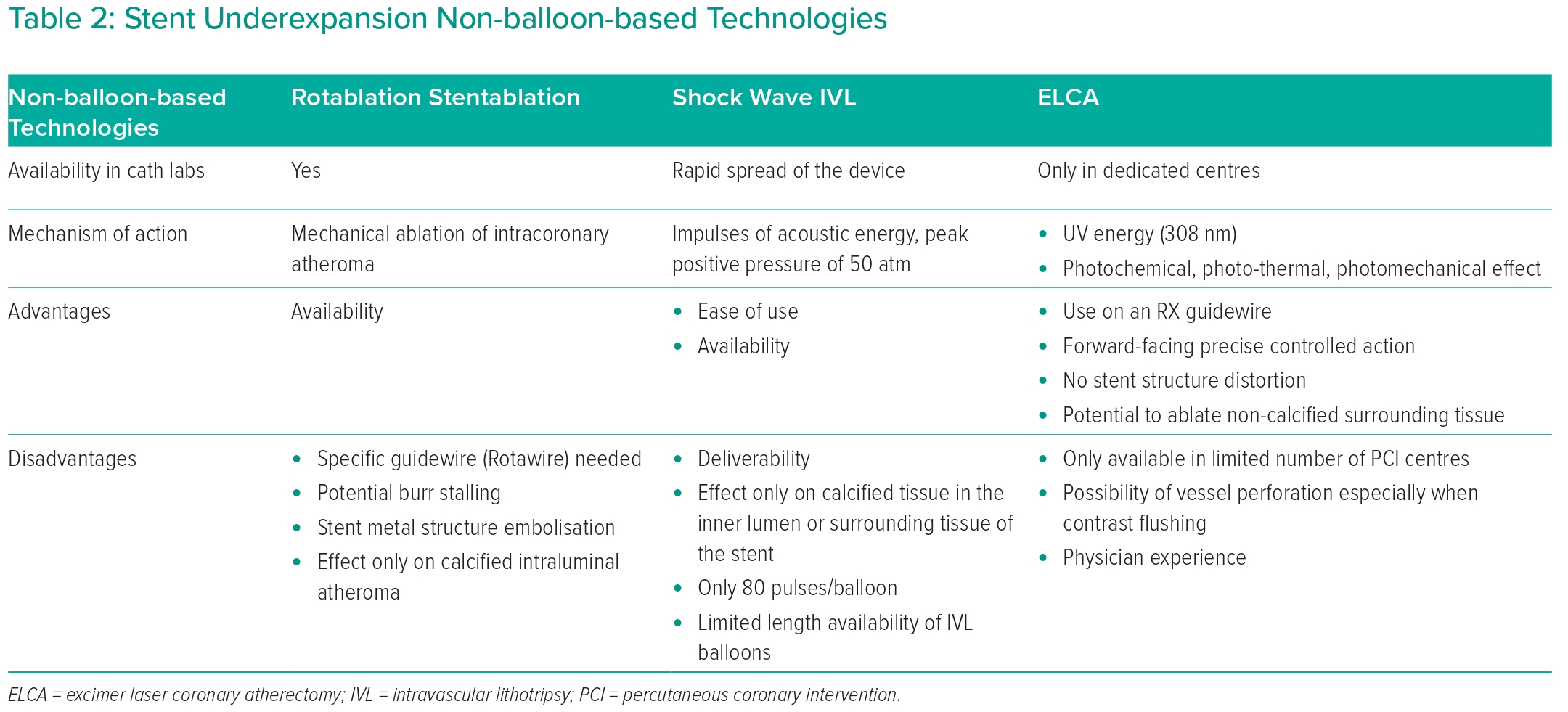
Excimer laser atherectomy (ELCA; Spectranetics) was performed (Figure 2A) using a 0.9 mm X-80 catheter. Based on prior major difficulties and OCT images, maximal 80 mJ/mm2 energy was selected with a pulse repetition rate (PRR) of 80 Hz. A total of 7,000 pulses were delivered over 10 trains. During the first seven trains, only saline was administered. During the last three trains, contrast injection was used to enhance efficacy, although it is currently off-label indication. After lasing, no OCT run was performed before re-dilatation attempt to spare contrast media. We opted for OPN NC balloon because it behaves as a regular NC balloon at lower pressures (<30 atm), but due to its structural strength, super high pressures can be applied to enhance dilatation efficacy (4.5 × 10 mm; SIS Medical; Figure 2B) if needed. At slowly increased pressures of 28–40 atm, the under-expanded stented segment tight stenosis responded well; consequently, good balloon expansion could be achieved. Total expansion of the balloon could only be achieved at 40 atm. Post OPN NC balloon use, OCT confirmed an excellent result with no dissection plane or vessel perforation present (Figure 2B [Ba OCT image] and Supplementary Material Video 3). OCT demonstrated a significant decrease in neointimal material and an MSA of 9.79 mm2. At the site of lasing and super high-pressure ballooning, an Onyx 5 × 30 mm stent (Medtronic) was placed at rated burst pressure (RBP) to avoid late recoil. More distal RCA segments were treated with appropriate-sized DES. Post-dilatations with adequate size NC balloons were performed in all treated segments. Final angiogram (Figure 2C) and OCT (Figure 2C [Ca, Cb OCT images] and Supplementary Material Video 4) revealed an excellent result. Final MSA at the lesion of interest was 12.2 mm2. Dual antiplatelet therapy (aspirin and clopidogrel) was maintained after MULTIPLATE ADP test effectiveness.
Six-month follow-up angiography demonstrated no recoil or in-stent restenosis. One and two-year clinical follow-up did not reveal any ischaemic symptoms.
Discussion
In a relatively high number of PCI cases, operators choose a direct stenting strategy. In the last two decades (the stent era), if an angiography result was misinterpreted or there was a lack of intravascular imaging (IVI), stents were placed in lesions without proper lesion preparation. If, after direct stenting, the stent structure does not open and stent under-expansion is >50%, the likelihood of early or late stent thrombosis is very high, with the potential of fatal outcome.
Intravascular Imaging
Intravascular Ultrasound, OCT
Intravascular ultrasound (IVUS) and OCT best characterise the full thickness of the native or stented arterial wall. The 2021 ACC/AHA/SCAI Guideline recommends using intracoronary imaging for guidance of the procedure in complex scenarios (class 2a recommendation, level of evidence B).1 IVUS can help evaluate calcium distribution or deposits even in deeper tissues of the vessel. OCT uses infrared light to provide higher-resolution images that can help characterise the calcium extension and thickness in the vessel wall. If pre-PCI OCT assessment is performed, three parameters have been identified to predict stent under-expansion: arc of calcium ≥180° (1 or 2 points); calcium length >5 mm (1 point); and calcium thickness >0.5 mm (1 point).2 Three or fewer points would be considered mild or moderate calcification, more than three points would indicate severe calcification. On IVUS assessment the four features that can predict stent under-expansion are the length of superficial calcium >270° (≥5 mm), circumferential 360° calcium, presence of calcified nodules, and relatively smaller calibre vessel (≤3.5 mm).3 All of these would account for 1 point; above a combined score of 2, severe calcification is verified and plaque modification is strongly advised before stenting.
In the present case, OCT was performed, which confirmed heavy calcification and the combined score derived from OCT was 4 points, which would translate to severe calcification in the vessel wall surrounding a partially crushed stented segment.
OCT for Guiding Stent Under-expansion Treatment
OCT intracoronary imaging can provide high-resolution images (12–15 µm) of the inner lumen, stent structure and intima media, and it is a very useful tool to describe pre- and post-ELCA effectiveness. OCT helped us understand the anatomy and stent structure allocation at the lesion site (from 12 to 6 o’clock, heavy calcium appearance, from particularly 6 to 12 o’clock, multiple layers of stent material indicating crushed stent).1,2
Plaque and Calcification Modifying Techniques and Tools
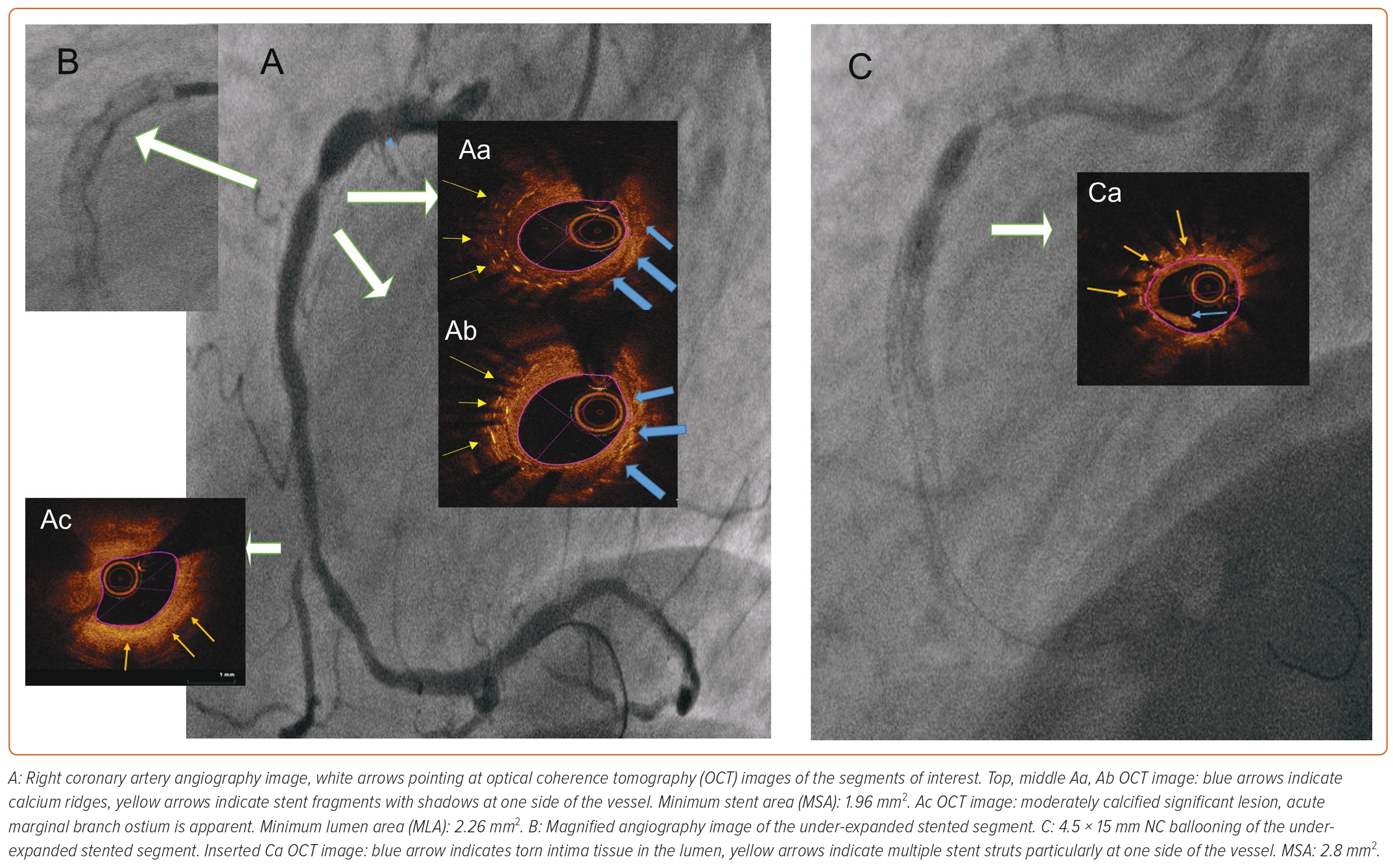
Balloon-based Techniques
Non-compliant Balloons
There are times when stent expansion is insufficient, even at higher inflating pressures than rated burst pressure (RBP), due to surrounding calcification or fibrotic tissue. Stent thrombosis is the most feared consequence of stent under-expansion and holds the potential of acute, subacute or late stent thrombosis, which can lead to fatal outcome.4,5
For stent post-dilatation, NC balloons are the first-line treatment option. NC balloons can tolerate higher inflating pressures and deliver more homogenous expansion compared to regular compliant balloons. However, due to non-homogenous balloon expansion or calcium presence, balloon rupture can occur, potentially causing dissection outside the prior implanted stent or even vessel perforation. In a non-negligible number of cases, NC balloon use is not enough for proper stent expansion (Table 1).
Cutting Balloons
A cutting balloon is an NC balloon with atherotomes attached to the surface of the balloon longitudinally. The downside of the cutting balloon is rigidity caused by the blades attached to the surface, thus crossability and deliverability even before use limit the success of this technique in native or stented vessels. For stent under-expansion or malapposition there are few reports of cutting balloon use.6 The downside is that underlying structures cannot be modified, thus cutting balloon dilatation should only be considered if other plaque-modifying devices are absent (Table 1).
Scoring Balloons
A scoring balloon is a semi-compliant balloon with sharp scoring elements on the surface of the balloon. These elements allow focal application of force throughout the inflation, thus permitting focal force application on the surface of the calcified plaque.7 There is no direct comparison between cutting and scoring balloons, but when compared to cutting balloons, deliverability is better and the chance of coronary dissection is lower. There is only one reported case of stent under-expansion, by Kashiwagi et al. In this report, stent restenosis (ISR) driven newly implanted everolimus-eluting stent under-expansion was observed. OCT-guided scoring balloon dilatation was performed, but residual stenosis was still present at the end of the procedure (Table 1).8
High-pressure Balloons
The super high-pressure balloon (OPN NC, SIS Medical) is a rapid-exchange non-compliant balloon that, due to its twin-wall technology, can bear very high pressures (RBP 35 atm) with a unique inflator device that can hold 40 atm. This device is primarily dedicated to calcified lesion preparation, where conventional NC balloons fail. There are two reports of OPN NC use in stent under-expansion. Raja et al. reported a case of stent under-expansion treatment, but no imaging was performed and NC balloon use at 20 atm was attempted for an unknown length of time before OPN NC, which at only 30 atm quickly solved the issue.9 One case report of OPN NC ballooning attempt for severe stent under-expansion is available, but application of super high pressures as a therapy alone was not successful, probably due to a lack of modification of the surrounding calcified, fibrotic tissue (Table 1).10
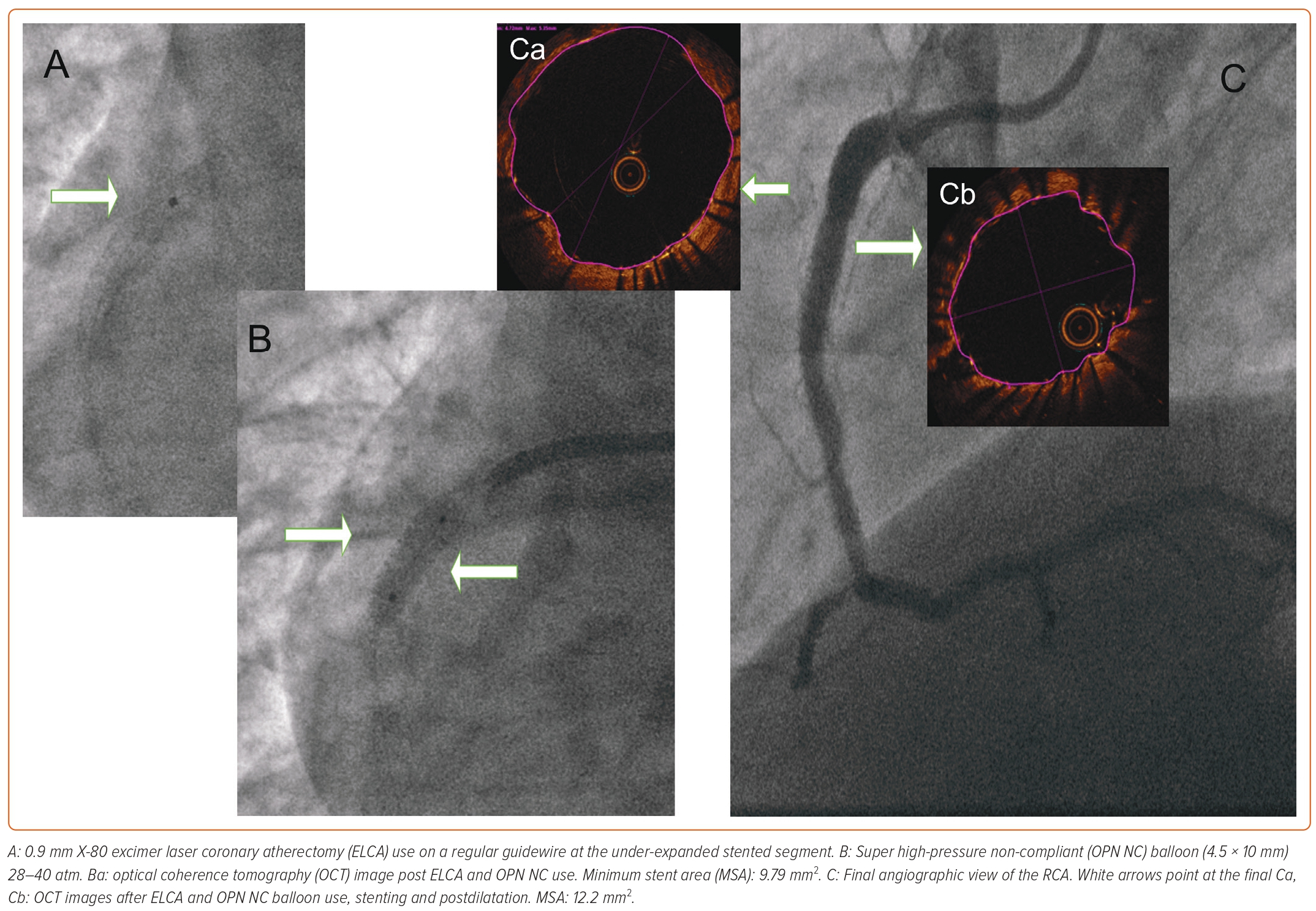
Rotational Atherectomy–Stentablation
Rotational atherectomy (Rotablator or RotaPro) uses a high-speed rotating burr that on the front surface is covered with diamond. Rotablation can mechanically ablate hard calcified atheroma and non-calcified pliable tissue is moved aside during ablation. If conventional balloon, NC balloon angioplasty fails, rotational atherectomy–stentablation has been a viable option, but there are numerous pitfalls. The lumen it can create is small, especially if a large-sized diameter vessel is ablated. Metal fragments can embolise to the microvasculature during ablation. Furthermore, the vibration effect in most cases is not enough to facilitate further balloon preparation of the lesion. Even if the burr crosses, the return holds the potential of being stalled.11 In a larger single-centre database of 20 patients, the procedural success was high, but the 1-year major adverse cardiac events (MACE) rate was 40%, which was mainly driven by target lesion revascularisation (TLR) (30%).12 Data is not encouraging for long-term success (Table 2). In the present case, stent rotablation was attempted, but after initial burr crossing, the return was difficult. However, the burr could be retrieved with no additional wire or parallel small-diameter balloon involvement and the procedure was aborted.
Shock Wave Intravascular Lithotripsy
An evolving therapy, shock wave IVL has emerged in the last 5 years. It uses acoustic energy to crack the calcified plaque by creating microfractures. When it is delivered, the balloon is inflated at 4 atm. It uses impulses of mechanical energy at a frequency of 1 pulse/second. These positive shock waves can generate a peak positive pressure of 50 atm with a duration <5 μs. This vibration effect can crack calcium in the superficial and deeper layers of the vessel wall, while elastic tissue is spared.13 Its relative ease of use in native calcified coronary vessel preparation has encouraged operators to treat stent under-expansion, although it is currently off-label indication. As it would be difficult to compare different modalities due to patient population differences and there is no randomised data, we must rely on case series and retrospective analysis on its use in conventionally undilatable stents (Table 2).
In a French multicentre registry, balloon advancement into the lesion intended to treat was 92.3%. The angiographic success rate was 73.1% and the procedural complication rate was 7.8%.14 In the CRUNCH registry, the procedure was considered successful if the residual stenosis was less than 50% and device delivery could be performed without complication. The device success rate was 92%, luminal gain was significant (not clear) and the device could not be delivered in only one case.15 In light of these registry data, the procedural success range is 73–92%, but residual stenosis and device deliverability could limit the final outcome and long-term success of IVL-treated conventionally undilatable stents (Table 2).
Laser Atherectomy (Light Amplification by Stimulated Emission of Radiation)
Excimer laser coronary atherectomy (ELCA) was approved by the Food and Drug Administration in 1992. ELCA uses electromagnetic energy to stimulate electrons and to elevate them into a higher state of energy. As the electrons return to their own baseline level, protons are released, resulting in monochromatic light emission. ELCA uses ultraviolet (UV) energy (wavelength of 308 nm) delivered by a xenon-chlorine laser catheter. Pulse frequency can be 25–80 Hz, fluence 30–80 mJ/mm2. By its photochemical effect, UV light hits the tissue for 135 billionths of a second. Billions of molecular bonds are fractured per laser pulse. Because of the absorption of UV energy, molecular-level vibration occurs and vibration heats intracellular water. Water vaporises, cells rupture and vapour bubbles are produced. Expansion and collapse of bubbles breaks down the tissue and clears by-products away from the tip of the catheter (Table 2).
The above-described balloon technologies could fail due to lesion resistance around the implanted under-expanded stent. Rotablation, as demonstrated above, has poor initial and long-term outcome and IVL technology can only be successful if the IVL balloon can be delivered. We assumed that if rotablation was unsuccessful in the present case, IVL balloon would not pass into the lesion.
ELCA, as described above, with the emitting fibres located at the tip and with precise forward-facing action and rapid exchange technology on a regular guidewire, could remain the best available option to modify persistent resistant plaque surrounding unopened stents.16–18
ELCA technology is only available in a limited number of PCI centres, reflected in the British registry, which between 2006 and 2016 identified 1,471 (0.21%) ELCA cases of 686,358 PCI procedures. In this registry ELCA procedure was driven by higher age, BMI, numerous lesions, CTO, restenosis treatment, longer stents, prior MI, CABG or PCI, showing more complex or previously attempted treatments with unsatisfying outcome. MACCE was not significantly higher in ELCA-treated patients, although higher odds of perforation, dissection and reintervention were documented.19 In the same database, only 0.02% of cases were treated with a combination of ELCA and ROTA (RASER). These cases were associated with more complex baseline characteristics. No change in overall MACCE was observed, but increased risk of shock, arterial complications and slow flow was observed.20
We know that it is not only the amount of calcium, but also the resistant scar and fibrotic tissue surrounding the implanted stent or multiple layers of stent layers that cause failure to perform sufficient balloon dilatation, rotablation and IVL of these prior stented lesions.
In our patient who was treated after analysing historic retrospective data on a relatively small number of case series of patients, we tried to avoid the combination of ELCA and ROTA due to procedure complexity and higher risk of slow flow, dissection and perforation.20 We investigated a potentially simple option that had not been reported before: the use of only ELCA with a 0.9 mm X-80 catheter followed by super high-pressure OPN NC balloon use.
Rawlins et al. published a review article on ELCA technology and it is well described that in under-expanded stented segments, ELCA could be the treatment of choice as it has the potential to ablate surrounding resistant atheroma without stent distortion. It is important to use a 0.9 mm catheter rather than a larger size catheter to minimise bubble formation.21 Latib et al. proved in the ELLEMENT study of 28 patients that under-expanded stent treatment with ELCA and contrast injection within the stented segment was highly successful (96.4%). Periprocedural myocardial infarction (7.1%), transient slow flow (3.6%) and ST elevation (3.6%) were acceptable, making it a feasible interventional option.22
Contrast Enhancement of Laser Efficacy
Contrast injection can facilitate the ablative effect of lasing.17,23 Karácsonyi et al. demonstrated in a report that contrast injection during lasing can amplify the ablative effect of lasing, and with the mandatory precautions, an excellent outcome can be achieved.18 In a recent review by McQuillan et al., the use of ELCA catheter for undilatable stents with or without contrast use is discussed and recommended in certain situations to achieve a good outcome.21
Considering all the data available in the literature, we planned OCT-guided ELCA with contrast flushing followed by OPN NC balloon to the resistant lesion. To simplify and shorten the procedure, it is reasonable to perform ELCA followed by OPN NC balloon in cases of severe stent under-expansion guided by intravascular imaging.
Honton and Monsegu published a review of the use of IVL in calcified lesions and although in the text and flowchart they advised the use of rotational or orbital atherectomy in cases of failure to deliver, as described above, these options are often not successful in cases of stent under-expansion.24 The authors agree with Vera-Vera et al., who had some specific suggestions to the proposed algorithm, namely the use of ELCA in moderate/severe calcified lesions.25 The authors believe that in the specific subset of patients presenting with severe stent under-expansion, after failure of NC ballooning and rotablation, it would be reasonable to use ELCA subsequently followed by the use of super high-pressure balloon. As described above OPN NC balloon behaves as a regular NC balloon under 30 atm. If the pressure rises at a slow rate by the inflator device under fluoroscopy, visual guidance can help, and the pressure can come down when the vessel responds well to avoid potential vessel perforation. These algorithm options are proposed for highly calcified, resistant lesions, but can be partially applied in stent under-expansion treatment.24,25 Once the vessel preparation is complete, the lesion can be treated according to guidelines as it was in the present case.26,27
Conclusion
As randomised data is lacking, we rely on retrospective analysis of cases with severe stent under-expansion. Considering the available data presented the authors strongly believe in the use of ELCA followed by OPN NC for treatment of severe stent under-expansion. The use of intravascular imaging, specifically OCT, is highly recommended in stent under-expansion treatment. 
Clinical Perspective
- Treating multiple layers of focally under-expanded crushed stent layers is crucial for avoiding stent thrombosis, thus a catastrophic outcome for the patient.
- There are some reports of using rotational atherectomy for under-expanded stents but retrieving the burr after crossing could result in failure. The cracking effect of rotational atherectomy might not be enough to facilitate further balloon angioplasty in a large-diameter vessel.
- Deliverability of the intravascular lithotripsy balloon or balloon-related issues can decrease the success rate of intravascular lithotripsy balloon in cases of severe stent under-expansion.
- Excimer laser coronary angioplasty combined with super high-pressure balloon, guided by optical coherence tomography can be a good option in treating under-expanded, crushed multiple layers of stents if available.