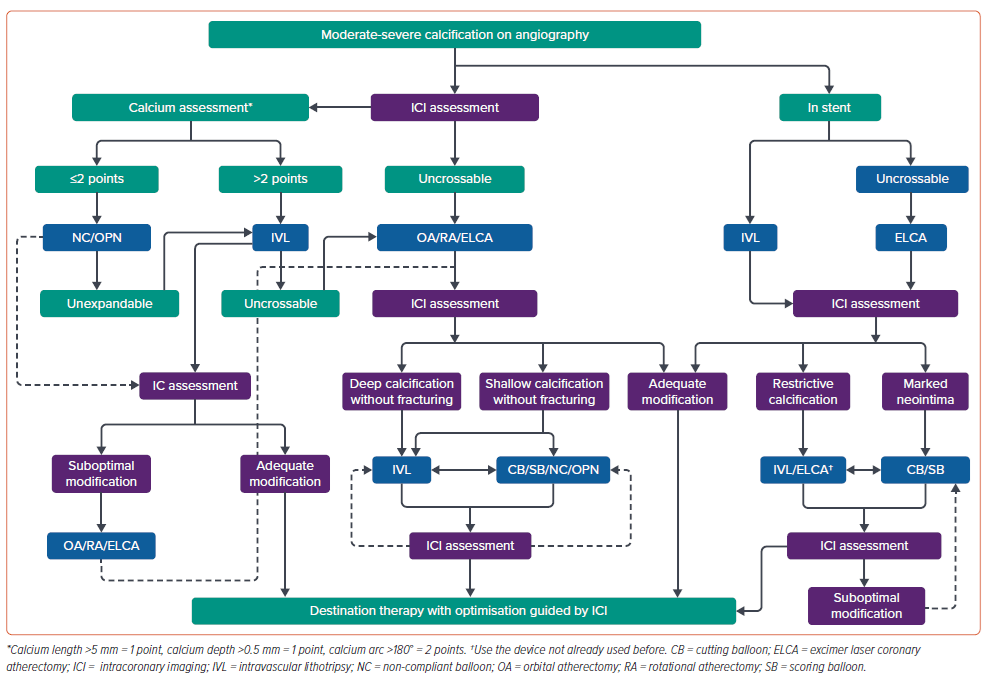
Coronary artery calcification is frequently encountered during percutaneous coronary intervention (PCI) and its presence is associated with a higher risk of stent under-expansion which has been associated with long-term risk of target lesion failure (TLF) and target lesion revascularisation (TLR).1–5 There are several different calcium modification strategies available, whether they be balloon-based devices (cutting/ scoring and intravascular lithotripsy) or atherectomy devices (orbital atherectomy, rotational atherectomy, excimer laser coronary atherectomy) that are established for the modification of coronary calcification.6–15 Each strategy applies a different mechanism of action for calcium modulation and consequently the potential to combine modifying tools may offer synergistic advantages over device monotherapy (Figure 1).
This article will focus on reviewing the evidence for the use of multiple calcium modification techniques and examine whether there really is any such synergistic effect of combining these tools. Intracoronary imaging, which in our opinion is essential to guide therapy in these cases, provides an understanding of the impact of each device on calcified lesions and determines whether further methods of modification are required.16–18 Figure 1 provides a suggested algorithm for the management of complex coronary calcification and given the essential nature of intracoronary imaging to guide the procedure, imaging features at each point of the decision tree.
Rotational Atherectomy and Cutting Balloons
The combination of rotational atherectomy (RA) with cutting balloon (CB), termed rota-cut, has conceptual advantages over use of a single RA strategy alone.19,20 RA is effective at modifying superficial calcification which often limits the delivery of bulky balloons, such as CB, but often the size of RA burr is limited by either guide catheter or vessel diameter.21 The use of CB following RA means that they are more easily delivered but also that the focal forces from the CB are delivered to already partially modified plaque.21
In 2016, Li et al. published a randomised pilot study to evaluate the efficacy of a rota-cut strategy compared with RA with conventional balloon use. The study included 71 patients, the majority of whom were men and had a mean age over 70 years with significant cardiovascular comorbidity (73% diabetes, 77.5% hypertension, 59.1% smokers, 53.5% dyslipidaemia, 18.1% chronic kidney disease).22 Interestingly, a significant proportion (42.2%) had left ventricular dysfunction (ejection fraction <50%).22 A significant proportion of these cases were in the left main stem (14.1%) or left anterior descending (LAD; 60.5%), although, surprisingly, given this, the mean stent diameter was around 2.75 mm for both arms.
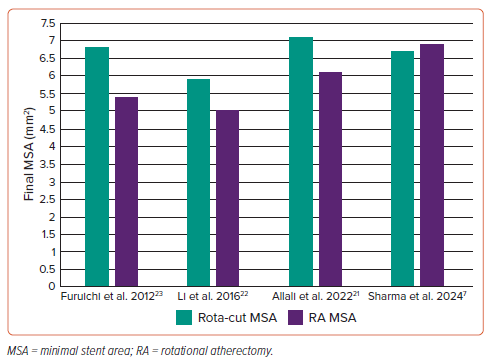
The concept that the rota-cut strategy would limit large burr use was not borne out in this study, given that there was no difference in mean burr size between the groups (both around 1.5 mm). It is possible that the performing clinicians simply did not want to increase the burr size or were not able to in the RA alone group. Interestingly, this study demonstrated that the minimal stent area (MSA) and the acute area gain were both higher with the use of rota-cut compared with RA and standard balloons alone (5.9 ± 1.7 mm2 versus 5.0 ± 1.4 mm2 ; p=0.021 and 4.5 ± 1.5 mm2 versus 3.8 ± 1.5 mm2 ; p=0.035; Figure 2).22 The addition of CBs did not seem to alter the procedural safety as the rates of dissection or slow/no flow were similar in both groups (8.3% versus 11.1%; p=0.720 and 8.6% versus 8.3%; p=0.972, respectively).22 Importantly, at 1-year follow up the rota-cut cohort had improved outcomes with lower TLR (5.7% versus 22.2%; p=0.046).22 Given the relatively small study size, these data should be interpreted cautiously but they certainly point to the possibility that rota-cut is as safe as, and potentially more efficacious than, RA with standard balloons.
Similarly, Furuichi et al. retrospectively identified 10 patients treated with rota-cut and compared them with 15 in whom RA was used with standard balloons. Much like the previous study, this cohort was an elderly group, the majority of whom were men with a high frequency of cardiovascular comorbidity.23 Again, there was no difference in the final burr size between the groups but also, again, there was a significant increase in the final MSA in the rota-cut cohort compared with the RA group (6.80 ± 1.27 mm2 versus 5.38 ± 1.89 mm2 ; Figure 2).23 There were no adverse events at 6-month follow-up but clearly the study is too small to make a proposal that clinical endpoints could be improved by larger MSA although this has some plausibility, despite the data from ILUMIEN IV suggesting modest increases in MSA did not improve endpoints.24
More recently, the PREPARE-CALC-COMBO study included 110 patients treated with a rota-cut strategy and compared both lumen gain and stent expansion against a historic cohort of patients treated with either RA or CB alone (PREPARE-CALC).21 Much like the previously published data, this cohort was elderly with significant comorbidity and predominantly male. Again, the rota-cut strategy demonstrated a significant improvement in final MSA compared with either the RA or balloon strategies (7.1 ± 2.2 mm2 versus 6.1 ± 1.7 mm2 versus 6.2 ± 1.9 mm2 , respectively; p<0.004; Figure 2) and the lumen gain was also significantly better for the rota-cut treated cohort compared with either the RA or balloon only groups.21 While these data are promising, it is important to note that this cohort was compared with a historic group, which does somewhat limit the interpretability of the study. The 9-month follow up demonstrated 4.5% mortality (1.8% cardiac) with 6.3% TLR which, considering the multimorbid elderly population with severe coronary calcification studied, suggests that rota-cut offers good durability.21
The latest study, the ROTACUT trial, by Sharma et al., has recently been published and randomised patients with severe coronary calcification to either a rota-cut or an RA and non-compliant balloon strategy. Again, the baseline demographics were similar to previous studies; elderly, predominantly male with significant comorbidity.25 Importantly all angiographic and Intravascular ultrasound (IVUS) findings were reviewed by an independent blinded core lab. Unlike the previous studies, ROTACUT demonstrated no improvement in MSA with rota-cut as compared with RA plus non-compliant balloons (6.7 ± 1.7 mm2 versus 6.9 ± 1.8 mm2 ; p=0.685; Figure 2).25 At 30 days, there was one target vessel revascularisation, but this was in a patient planned for rota-cut that crossed over to RA plus noncompliant balloon treatment.
These randomised data demonstrate that while rota-cut is safe it may not be associated with improved outcomes over an RA and non-compliant balloon strategy. Clearly, compared with PREPARE-CALC-COMBO, the ROTACUT study is much less likely to be influenced by operator bias and the limitations of the comparison with the historic control arm in PREPARECALC-COMBO. While it is disappointing that results of the ROTACUT study were discordant with the earlier hypothesis that rota-cut would lead to larger MSA, it does not alter the fact that in selected cases the combination does provide better lesion preparation. For this combined strategy to be adopted routinely however, much larger studies would be required to define whether it can improve clinical endpoints.
Rotational or Orbital Atherectomy in Combination with Excimer Laser Coronary Atherectomy
The combination of RA and excimer laser coronary atherectomy (ELCA), often termed RASER, has been described as an effective strategy for managing non-crossable calcific lesions.10,26–30 In this combination therapy, ELCA has the advantage that it can be delivered on a standard 0.014" guidewire which is much easier to deliver than either the 0.009" rotawire required for RA or 0.012" viper wire required for orbital atherectomy (OA). ELCA creates a channel to either permit delivery of a microcatheter to facilitate delivery of the rota/viper wire or increase the likelihood of freewiring with the dedicated wire.10,26–30.
A single centre analysis from the UK of 50 ELCA cases included 11 (22%) using the RASER technique.31 ELCA was able to cross the lesion in all 11 RASER cases but there was one perforation in the ELCA cohort although this was attributed to the use of RA.31 Similarly, in the cohort of 119 ELCA patients by Badr et al., 12 (10.0%) involved a combination therapy with RA, where the majority (11, 91.7%) involved ELCA first followed by RA.32 A RASER strategy was most frequently used in calcified lesions (24%) with less frequent use in saphenous vein grafts (15.0%), chronic total occlusions (6.2%) and in-stent restenosis 6.7%.32 While this analysis did present angiographic outcomes and complication details these were not split by whether RA was used.
The largest reported RASER series is presented from the British Cardiovascular Intervention Society database, which included 153 cases (0.02% of the total PCI cases in the database) between 2006 and 2016.33 Unsurprisingly, given the complex nature of lesions treated with RASER, this study demonstrated that these patients had higher rates of cardiovascular comorbidity, previous revascularisation and left ventricular dysfunction.33 Again, the type of lesions that were treated with RASER tended to be more complex than the rest of the cohort as demonstrated by the higher frequency of both chronic total occlusions and use of intracoronary imaging as well as the longer final stent lengths.33 The use of RASER was also associated with a higher rate of CB usage (13.1% versus 3.2%, p<0.01), thus demonstrating the operators perceived benefit of multicombination therapies for the modification of complex calcification.33 In terms of outcome RASER was associated with a higher frequency of complications on unadjusted analysis and after multivariable analysis RASER remained significantly associated with the induction of shock (OR 9.66, 95% CI [3.44–27.06]), slow flow (OR 3.50, 95% CI [1.29–9.55]), and arterial complications (OR 3.23, 95% CI [1.58–6.61]).33 Overall, hospital major adverse cardiovascular events (MACE), major bleeding or death were not increased with the use of RASER.33
The data for the use of RASER are generally based on small populations of patients with complex coronary artery disease and increased comorbidity but the evidence available suggests that this strategy is potentially safe and effective when used by experienced operators in selected patients.
Rotational or Orbital Atherectomy in Combination with Intravascular Lithotripsy
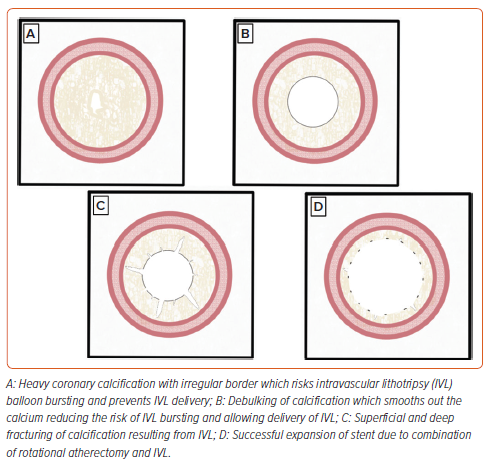
Like the RASER technique, the combination of RA/OA with intravascular lithotripsy (IVL) uses the crossing ability of one strategy (RA/OA) to allow the delivery of a more difficult-to-deliver device (IVL). The RA/OA ablates the superficial calcium creating a channel for delivery of the IVL which then further modifies the superficial calcium and modifies the compliance of the medial wall calcium (Figure 3).34–36 Unlike ELCA, RA/OA and IVL are widely available and therefore there has been considerable interest in combining these strategies for the management of complex coronary calcification. These strategies have been labelled rotashock/rotatripsy and orbitalshock/orbitatripsy in the literature, but for the remainder of this manuscript we will use the terms rotashock and orbitalshock.
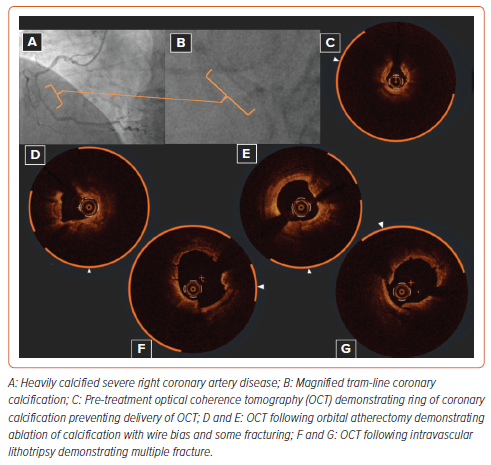
Rotashock has been described and shown to be efficacious in several case reports and case series.37–41 These cases demonstrate that this strategy, much like other combination strategies already descried, tends to be used in elderly patients with multiple comorbidities. In the largest case series to date (seven), the average age was 76 and the majority of cases were performed in the LAD.39 In their cohort, there was one perforation, which was felt due to an oversized stent rather than as a result of rotashock.39 Similarly, there are many case reports and series demonstrating the potential utility of orbitalshock.42–44 Much like the rotashock case series, the largest orbitalshock case series was an elderly, predominantly male population with significant comorbidity.44 In seven of the eight cases in this series there was procedural success and there were no periprocedural complications or MACE within 30 days.44 Figure 4 demonstrates an example of orbitalshock with OCT appearances.
Takahashi et al. included 33 (30.3%) orbital/rotashock cases in their series of 109 patients treated with IVL.45 In this group, patients treated with orbital/rotashock had a higher frequency of previous MI and left ventricular dysfunction which resulted in higher rates of mechanical support in the combination group.45 In the combination cohort, the most frequent access site was femoral with a 7 Fr guide catheter, as opposed to the IVL group in which the procedure was more likely to be performed via the radial artery and a 6 Fr guide. This is surprising, as one of the perceived benefits of orbital/rotashock is that the IVL balloon negates the need for larger burrs and therefore these procedures could theoretically be performed using 6 Fr guides via the radial artery. In all except one of the patients in this cohort the PCI procedure was guided by intracoronary imaging which is essential to understand the impact any calcium modification strategy.16
This cohort demonstrated that there were high rates of device success (defined as successful completion of IVL without immediate complication) in the combination group (97.0%) and the IVL alone group (96.1%). Despite this, the overall rate of procedural success (defined as a residual stenosis of <30% with thrombolysis in MI [TIMI] 3 flow) was lower with 78.8% in the combination group compared with 98.5% in the IVL alone group (p=0.14). Interestingly in the combination group, there were six cases in which ELCA was used alongside orbital/rotashock, termed OA/RASERshock, again highlighting the potential synergy of all the calcium modification devices. In the combination group, there were two cases of dissection, one after ELCA and one after IVL. While longer-term results are required, reassuringly in this cohort at 30 days there were no cases of target vessel revascularisation and there were three MACE (one cardiac and one noncardiac death) in the combination group and two MACE in the IVL group.45
Similarly, in a cohort of 25 patients treated with orbital/rotashock the population was a predominantly male and elderly with significant cardiovascular comorbidity.46 Again, all of these cases were performed using atherectomy upfront to create a channel for the IVL balloon and the majority was performed with intracoronary imaging guidance (96%).46 Procedural success (defined as successful stent implantation, residual stenosis <30%, TIMI 3 flow and absence of significant complication or inhospital MACE) was achieved in 88%.46 Complete success was limited by one side branch loss and two in-hospital deaths (one cardiac arrest and one intracerebral haemorrhage).46
Sardella et al. published the largest rotashock series of 160 patients from 23 centres. Predictably, this was an elderly, predominantly male population with multiple comorbidity.18 In this cohort the majority of cases was performed with a single burr (73.1%) with 68.5% using a 1.5 mm or smaller burr.18 This demonstrates one potential advantage of a rotashock strategy over an RA with upsizing burr strategy in that it can potentially be performed via a 6 Fr guiding system which is usually simple through the radial artery.
In this study, there was a surprisingly low (52.5%) use of intracoronary imaging, acknowledged by the authors as being an essential technique to understand the degree of calcium modification.16,18 In this analysis the procedural success (residual stenosis <30% using quantitative coronary angiography) was excellent at 98.6% of cases.18 There were three (1.9%) patients who had a dissection of a grade C or higher, four (2.5%) cases of perforation and eight (5.0%) cases of slow/no flow.18 Freedom from inhospital MACE was 98.7% which is impressive considering the complexity of these cases; a sixth involved treating the left main stem and the median lesion length was 35.1 mm (± 21.3 mm).18 The data thus far on orbital/ rotashock demonstrate that these techniques have a high likelihood of success and relatively low complication frequency, particularly given the complexity of these lesions.
In our series of 36 patients, the majority of whom were elderly (median age 75 years (IQR 70–79 years) and male [80.6%]), there was a high intracoronary imaging frequency (88.9%).47 This analysis demonstrates one of the potential advantages of rotashock strategy; the use of IVL reduces the need for multiple (86.1% single burr), larger burrs (75.0% ≤1.5- mm burr) and as such the majority (80.6%) of these cases were performed using radial access alone.47 The cases included in this analysis are likely to be more complex than the cohort from Sardella et al., given that the median lesion length was 60 mm, a third involved the left main and a quarter involved a two-stent bifurcation strategy. Despite this, the clinical outcomes were good with no immediate complications other than two relating to the access site. Furthermore, the median MSA was 7.7 mm2 and at a median 942 days of follow-up there were two cases of target vessel revascularisation.47 However, these data are limited by their size and the single centre nature but demonstrate the potential of rotashock to treat complex calcific disease in conjunction with intracoronary imaging. Further data are required to evaluate the longer-term durability of these results and, in particular, randomised data comparing the use of combination therapy against the use of a single device, either atherectomy or IVL alone.
Intravascular Lithotripsy in Combination with Excimer Laser Coronary Atherectomy
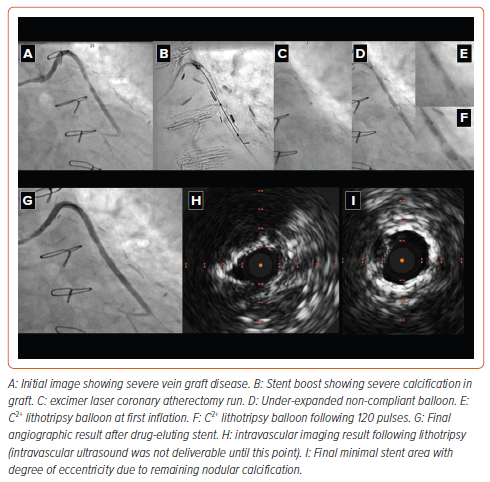
Much like orbital/rotashock, the combination of ELCA with IVL (termed LASERSHOCK) uses the complimentary mechanisms of action of these devices, whereby ELCA provides a channel to allow delivery of the more bulky IVL balloon.48 In particular, there are situations where orbital/ rotational atherectomy are not well suited or where the specific wires required to use these atherectomy devices will not pass a lesion and, as such, the use of an LASERSHOCK strategy might be preferable.48 Despite the potential utility of this strategy there are limited data in the literature. There is one case report. In this case the patient had a proximal lesion treated with drug-eluting stents and came back for intervention to a more distal lesion which would not expand with balloon angioplasty and the IVL balloon would not cross the lesion.49 Given the recent stents the operators opted to use ELCA rather than orbital/rotational atherectomy to create a channel to deliver the IVL balloon.49 While there was a good final result, this is only a single case report and further data are now required to prove that this strategy with conceptual benefits is useful in practice. Figure 5 demonstrates a case in which ELCA shock was used to treat a calcified vein graft.
Conclusion
Despite the development of multiple calcium modification strategies and the increasing use and understanding of intracoronary imaging, coronary calcification still limits the procedural success and longer-term outcomes that interventional cardiologists can achieve for their patients. The various combinations of these technologies, with their different mechanisms of action, may be synergistic and, as such, could provide improvements in stent results in patients with severe coronary artery calcification. Further data, in particular randomised between single and dual strategies, are now required to fully evaluate these strategies.