Dr Everett’s research aims to understand the ventricular haemodynamics of mechanical circulatory support in acute MI (AMI). She shared her ongoing work examining how extracorporeal membrane oxygenation (ECMO) with concomitant Impella support (ECPELLA), can mitigate left ventricular (LV) injury and loading due to veno-arterial (VA) ECMO.
Dr Everett opened by reviewing the current mechanical circulatory support tools used in AMI cardiogenic shock (CS). VA-ECMO is widely used in CS. The impact of VA-ECMO on LV physiology depends on a number of factors, including ECMO speed, the degree of LV systolic dysfunction, mean arterial pressure and afterload. It has been shown that higher VA-ECMO flow generates higher afterload, which can worsen LV injury and lead to LV distension. Using pressure–volume (PV) loops, Dr Everett highlighted the differences in effects on LV pressure between ECMO, Impella and ECPELLA. VA-ECMO activation results in elevated LV pressures and volumes; the addition of unloading to ECMO, ECPELLA, mitigates these increases in pressures and volumes, although it is thought this configuration cannot achieve the same degree of unloading as isolated Impella. Dr Everett summarised that haemodynamics data support combining transvalvular unloading, known to offer myocardial protection in AMI, with VA-ECMO, which offers systemic perfusion in CS. It remains to be determined whether the combined unloading and VA-ECMO strategy indeed offers myocardial protection, and the best timing to implement unloading with VA-ECMO in AMI-CS. For her study, Dr Everett hypothesised that compared to ECMO alone, ECPELLA mitigates LV loading and injury when Impella is activated prior to ECMO and reperfusion in AMI.
Dr Everett’s work was performed in a porcine model of ischaemia–reperfusion injury (IRI) with mid-left anterior descending artery occlusion followed by reperfusion. Dr Everett compared IRI animals with three groups of mechanical circulatory support: VA-ECMO alone and two ECPELLA groups, one with Impella implanted before ECMO (Impella-ECMO) and one with Impella after ECMO implantation (ECMO-Impella). All three groups had VA-ECMO implantation prior to reperfusion.
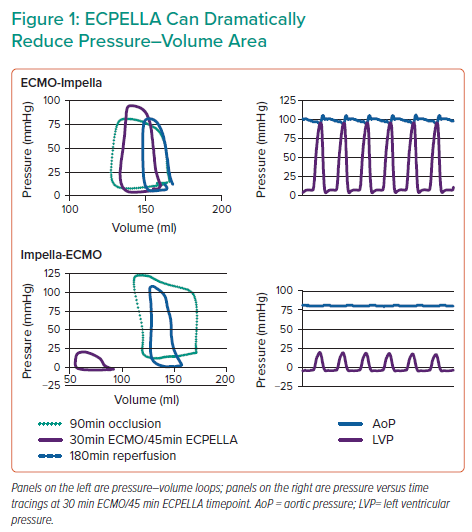
Dr Everett’s results showed that infarct size was increased with VA-ECMO alone, and this was comparatively reduced in both ECPELLA groups. Samples with the lowest infarct size had the lowest PV area (PVA), which was particularly true for the Impella-ECMO ECPELLA group. A comparison of PV loops revealed that Impella-ECMO also markedly reduced PVA and end-diastolic pressure (Figure 1), whereas ECMO-Impella did not. Furthermore, a subset of the Impella-ECMO group achieved large (≥50 mmHg) reductions in ventriculo-arterial (VA) uncoupling, an effect not observed in the other treatment groups (Figure 1). Dr Everett observed that these effects correlate with molecular signatures of cardioprotective pathways. Markers for the reperfusion injury salvage kinase (RISK) pathway, which is a known cardioprotective pathway, were evaluated. RISK pathway members Akt, extracellular signal-regulated kinase, and glycogen synthase kinase 3β were significantly upregulated in both ECPELLA groups.
Dr Everett concluded that ECPELLA with Impella initiated prior to VA-ECMO reduces infarct size and LV PVA and increases VA uncoupling and cardioprotective signalling in IRI. Together, these results suggest that early LV unloading coupled with VA-ECMO may mitigate LV injury in AMI-CS.