Spontaneous coronary artery dissection (SCAD) is defined as a non-traumatic and non-iatrogenic separation of the coronary arterial wall by intramural haemorrhage creating a false left main stem (LMS), with or without an intimal tear. The separation can occur between the intima and media, or between the media and adventitia. The resulting intramural haematoma (IMH) compresses the arterial LMS, compromising antegrade blood flow to varying degrees and producing subsequent myocardial ischaemia or MI.1 SCAD is a less common cause of acute coronary syndrome.
The true prevalence of SCAD is uncertain because the condition is often misdiagnosed. Recent series using careful diagnostic criteria that exclude iatrogenic, traumatic and atherosclerotic dissection suggest that SCAD may be the cause of acute coronary syndrome in up to 35% of cases of MI in women aged <50 years, and is the most common cause of pregnancy-associated MI.2 Pregnancy-related SCAD (P-SCAD) can occur at any stage from the first trimester up until 6 weeks postpartum.
We present a case of P-SCAD involving the left main coronary artery, complicated by cardiogenic shock, and describe a potential strategy to improve coronary perfusion in the setting of extensive IMH as a bridge to subsequent surgical revascularisation.
Case Report
A 30-year-old woman developed severe chest pain 1 day after uncomplicated vaginal delivery of her second child. The patient had developed gestational diabetes, but otherwise the pregnancy and delivery had been uncomplicated.
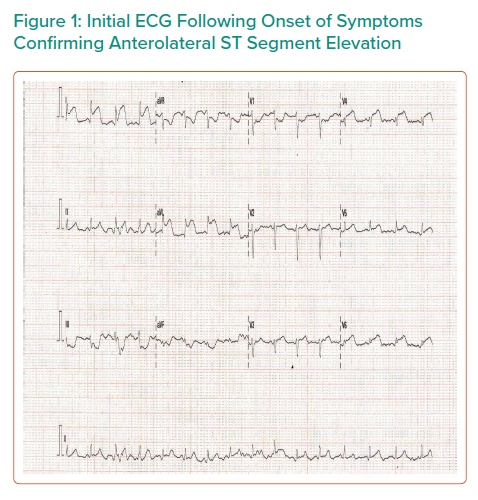
Initial ECG showed anterolateral ST elevation (Figure 1). The patient was transferred from the obstetric unit to the tertiary referral centre for emergency coronary angiography. On arrival she was initially haemodynamically stable, with a heart rate of 79 BPM, blood pressure of 117/86 mmHg and oxygen saturations of 98% on room air. Prior to transfer, the patient was administered 300 mg aspirin and 180 mg ticagrelor.
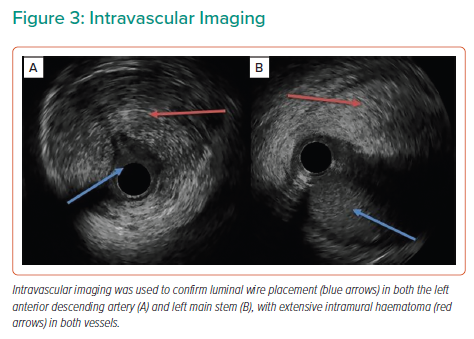
Coronary angiography via a right radial approach revealed a dominant right coronary artery (RCA) without disease, but the left anterior descending (LAD) and left circumflex (LCX) arteries were occluded proximally with angiographic evidence of a dissection plane extending back to the ostium of the LMS.
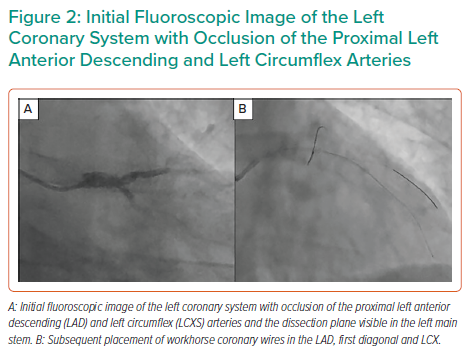
The LAD, LCX and first diagonal were wired with three low-tip-load workhorse wires (Figure 2), with luminal wire placement confirmed by intravascular ultrasound (IVUS). IVUS demonstrated extensive dissection and compressive haematoma throughout the length of the LAD extending to the LMS (Figure 3).
IVUS demonstrated that the mid- to distal LAD was approaching 3.0 mm in diameter and the proximal LAD was 3.5–3.75 mm in diameter. Given the extent of IMH, a 2.5 mm Wolverine cutting balloon (Boston Scientific) was used to decompress the haematoma in the mid- to distal LAD and a 3.0 mm cutting balloon was used in the proximal LAD. Balloon size was chosen based around a conservative 0.5 mm reduction in the IVUS external elastic lamina sizing. This restored Thrombolysis in MI (TIMI) II flow to the distal LAD, but, despite this, the patient developed cardiogenic shock (systolic blood pressure 70–80 mmHg) and acute pulmonary oedema requiring intubation and ventilation, inotropic support and insertion of an intra-aortic balloon pump (IABP).
Given the extent of dissection in the left coronary system, percutaneous revascularisation was unlikely to give a good short- or long-term outcome, and so the patient underwent emergency coronary artery bypass grafting (CABG) with vein grafts to the LAD and LCX. Coronary wires were left in the distal vessels during transfer to the cardiac theatre to guide the surgeon to the true vessel LMS and to maintain distal coronary flow by pinning back the dissection flap. The guide catheter was removed over the top of a coronary wire extension and the radial sheath was left in place during transfer.
At the time of the patient’s haemodynamic decompensation she had a profound metabolic acidosis (pH 7.09, pCO2 6.8, pO2 68.2 and lactate 7.1). Troponin T was also markedly elevated at >10,000 ng/l. Following intubation, ventilation and IABP insertion, there was a significant improvement in augmented systolic blood pressure (90–100 mmHg) and the metabolic acidosis improved (pH 7.23) prior to the patient’s emergency transfer to theatre.
The patient had a 3-day stay in cardiac intensive care, involving a 24-hour period of ongoing inotropic and mechanical circulatory support with an IABP. This was followed by a 7-day stay in coronary care for uptitration of heart failure pharmacological therapies, which included a β-blocker, angiotensin receptor–neprilysin inhibitor and mineralocorticoid receptor antagonist.
Postoperatively, left ventricular function ejection fraction (LVEF) was severely impaired (20%), but improved to moderate (LVEF 42%) 6 weeks after discharge. Late gadolinium enhancement demonstrated only partial viability in the LAD territory, but full viability and normal contractility of the LCX territory. The patient currently experiences no angina and has New York Heart Association (NYHA) Class I–II dyspnoea.
Discussion
The management of SCAD presents a significant clinical challenge, with the potential for percutaneous intervention to create serious complications and resultant adverse clinical outcomes. The condition is preferably managed conservatively without the need for coronary instrumentation; however, this can only be justified in the absence of ongoing ischaemia, ventricular arrhythmias, haemodynamic instability or the involvement of the LMS.3 In 2014, Vijayaraghavan et al. published a management algorithm for P-SCAD.4 This recommended that the revascularisation strategy should be based on both the clinical scenario and anatomical disease severity. CABG was considered preferable to percutaneous coronary intervention in LMS SCAD involving both the LAD and LCX or extensive SCAD affecting at least two proximal vessels when percutaneous coronary intervention is not feasible.
This case presents one of the most severe phenotypes of P-SCAD, with both extensive vessel involvement and cardiogenic shock. Coronary dissection and IMH can often be rapidly extended when attempting to insert wires into the distal vessel. Therefore, the use of an initial low-tip-load non-hydrophilic wire is preferable to avoid iatrogenic exacerbation of myocardial ischaemia. Previous studies have demonstrated a 71.4% success rate of such techniques and reserving escalation to hydrophilic wires for cases in which luminal guidewire placement is not possible.5
The use of cutting balloons to decompress IMH and restore flow has been well described, but this strategy has been largely adopted prior to drug-eluting stent placement.5–7 We demonstrate the ability for this technology to improve coronary flow in extensive SCAD as a bridge to cardiac surgery. It is vital that imaging be used in such cases to confirm luminal placement of coronary wires and assess the extent of dissection/haematoma.
For patients requiring emergency cardiac surgery, this case highlights the utility of IVUS-guided coronary wires to differentiate true and false LMS at the time of coronary grafting. This strategy was felt to be particularly useful by the operating cardiac surgeon in this instance and has the potential benefit of pinning back a proximal dissection flap and maintaining some degree of coronary flow during transfer to theatre. The only potential downside to this technique is the possibility of wire displacement during transfer to theatre. However, this was not observed in the present case and seems unlikely in an immobile and intubated patient.
Current literature on the use of mechanical circulatory support in SCAD complicated by cardiogenic shock is limited. IABP is the most widely reported form of support and can either stabilise the subsequent percutaneous revascularisation or act as a bridge to CABG. A systemic review of P-SCAD in 120 patients demonstrated the requirement for mechanical circulatory support in as many as 28% of presentations.8
Conclusion
P-SCAD is a rare but potentially life-threatening condition. We have described a particularly severe clinical example not suitable for percutaneous management alone and requiring surgical revascularisation. The techniques used demonstrate the potential to aid surgical management and improve clinical outcomes.
Clinical Perspective
- Pregnancy-related spontaneous coronary artery dissection (SCAD) is a rare and potentially life-threatening medical condition.
- Cutting balloons can be used to decompress intramural haematoma and improve coronary flow when adopting either percutaneous or surgical revascularisation strategies.
- In extensive SCAD requiring emergency surgery, consideration should be given to maintaining coronary wire position to allow for identification of true left main stem and help with subsequent surgical revascularisation.