Over the past decade, successive randomised trials positioned transcatheter aortic valve implantation (TAVI) as a preferred therapy in the majority of older patients with aortic stenosis irrespective of surgical risk.1,2 The excellent safety and efficacy of TAVI among older (≥75 years) low-risk patients forecasts its expansion towards younger low-risk populations. This trend has already found its way into the 2020 American College of Cardiology/American Heart Association guidelines that recommend an age threshold of ≥65 years as decision-making criterion for TAVI.1 In Europe, international guidelines maintain an age cut-off of ≥75 years because of unanswered questions regarding long-term benefits and drawbacks of TAVI versus surgical aortic valve replacement (SAVR) in younger patients.2
A key remaining issue of TAVI is the occurrence of conduction abnormalities (CA) and need for permanent pacemaker implantation (PPI). Both are more common after TAVI than after SAVR and have important implications for hospital length of stay, costs and clinical outcome. New left bundle branch block (LBBB) and PPI have been associated with lack of improvement in left ventricular (LV) ejection fraction and higher rates of heart failure, hospitalisation, PPI and mortality.3–5 The true incidence of TAVI-induced conduction disorders in younger patients and its clinical impact throughout their extended lifespan remains to be seen. Nonetheless, there is a need for innovative strategies to mitigate the burden and clinical consequences of this complication. The present review delves into the available information on the incidence and impact of new LBBB/PPI after TAVI with contemporary transcatheter heart valves (THV) and highlights the value of multi-sliced CT (MSCT) data interpretation to predict and prevent its occurrence.
Incidence and Impact of New Left Bundle Branch Block
In current practice, newly developed LBBB is observed in approximately 15–30% of patients. Although the rate of new LBBB has declined with the advent of new generation THV systems and improved implantation techniques, significant variability exists between device platforms. Recent data indicate that the frequency of new LBBB varies between 18% and 26% with the self-expanding Evolut PRO system (Medtronic), 14% and 19% with balloon-expanding SAPIEN valves (Edwards Lifesciences) and 11% and 13% with the self-expanding ACURATE neo device (Boston Scientific).6–11 The degree of variability in conduction abnormalities across THV platforms can be attributed to distinctions in the expansion mechanism, mechanical properties of the stent frame, implantation depth (ID) below the aortic annulus and amount of interaction with the LV outflow tract (LVOT). The self-expanding Evolut platform features repositioning/recapturing technology that increases interference with LVOT and conduction tissue, especially upon repetitive implantation attempts. Conversely, the self-expanding ACURATE neo system differs conceptually as it uses a two-step top-down release mechanism to minimise interaction and protrusion into the LVOT.
Most new LBBBs that develop in the acute phase of TAVI resolve over time while 35–45% persist at 1-year follow-up.5,12–14 Determinants of LBBB persistence are baseline intraventricular conduction delay (QRS duration), self-expanding valve platforms with a bottom-up deployment mechanism, ID and larger valve sizes.5,12–14 New LBBB emerging after hospital discharge is rare and has been reported in 2–4%.15 Hypothetically, ongoing degeneration of the conduction tissue and a continued radial expansive force imposed by the THV frame causing mechanical injury to the conduction system may be contributing factors.
The long-term clinical impact of new LBBB remains controversial. From a pathophysiological perspective, altered intraventricular conduction delay causes a loss of synchronised right and LV mechanical contraction leading to deterioration of left LV systolic and diastolic function. In line with this concept some studies found that new LBBB is associated with an increase in LV dimensions, no left ventricular ejection fraction recovery and a higher risk of adverse clinical outcome.16 Although some reports disputed the former, a systematic review and meta-analysis including >7,000 patients across 11 observational studies demonstrated that new LBBB predicts all-cause mortality, heart failure hospitalisation and need for PPI.17 Other studies identified new LBBB as a predictor of cardiovascular mortality and sudden cardiac death likely because of progression to high-grade or total AV block.18 Indeed, almost 10% of patients with a new LBBB will require a PPI during follow-up which highlights the importance of continued surveillance in a post-TAVI care programme with longitudinal assessment of clinical status, LV function (cardiac ultrasound) and electrocardiographic status.
Incidence and Impact of New Permanent Pacemaker Implantation
High-grade AV block occurs in approximately 10–15% after TAVI and 3–7% after isolated SAVR.19,20 The most powerful predictor of need for PPI after TAVI is the presence of baseline conduction injury. Patients with pre-existing right bundle branch block (RBBB; RR 3.12, p<0.001), bifascicular block (RR 2.40, p=0.002) and first-degree AV block (RR 1.44, p<0.001) seem at highest risk.21 Twenty-four-hour ECG monitoring before TAVI is a sensitive tool for detecting underlying conduction issues. It identifies up to one-third of patients who ultimately require PPI after TAVI.22 As with new LBBB post-TAVI, the degree of variability in PPI risk depends on the THV system used. The frequency of PPI is highest with self-expanding THVs that have a bottom-up deployment mechanism (Evolut system 12–18%; Portico/Navitor with FlexNav [Abbott] 9–19%), while lowest rates are reported after implantation of the balloon-expandable SAPIEN 3 Ultra (5–10%) and self-expanding THVs with top-down deployment mechanism (ACURATE neo2 system 7%).23-27 Overall, the indication for PPI is established <7 days after TAVI in almost all patients with around 50% becoming evident <48 hours after the procedure.28 New high-grade atrioventricular (AV) block after hospital discharge is infrequent but generally requires PPI. Ambulatory electrocardiographic monitoring is required to capture these events (8–10%) since most patients (60–80%) remain asymptomatic.29,30
While PPI is ultimately lifesaving and improves quality of life in many patients, it can impact patient outcomes and recovery as it involves an additional intervention (prolonged hospitalisation, complication risks) that induces unphysiological responses in the heart. Especially in patients with a longer life expectancy, right ventricular (RV) pacing induced ventricular desynchrony can contribute to a decline in LV function and overall cardiac performance over time. Faroux et al. found in a meta-analysis comprising 30 studies that PPI was associated with increased 1-year mortality (RR 1.17; 95% CI [1.11–1.25]) and heart failure hospitalisation (RR 1.18; 95% CI [1.03–1.36]) but not cardiac death.18 The lack of association between PPI and cardiac death may be explained by a limited follow-up duration in the included studies. Alternatively, the potential protective effects of PPI on the risk of life-threatening bradyarrhythmias may counterbalance the adverse effects on ventricular function. To restore the coordinated contraction of left and right ventricle in patients who are pacemaker-dependent, certain pacing strategies such as cardiac resynchronisation therapy may prove useful.
Multi-sliced CT Data Interpretation
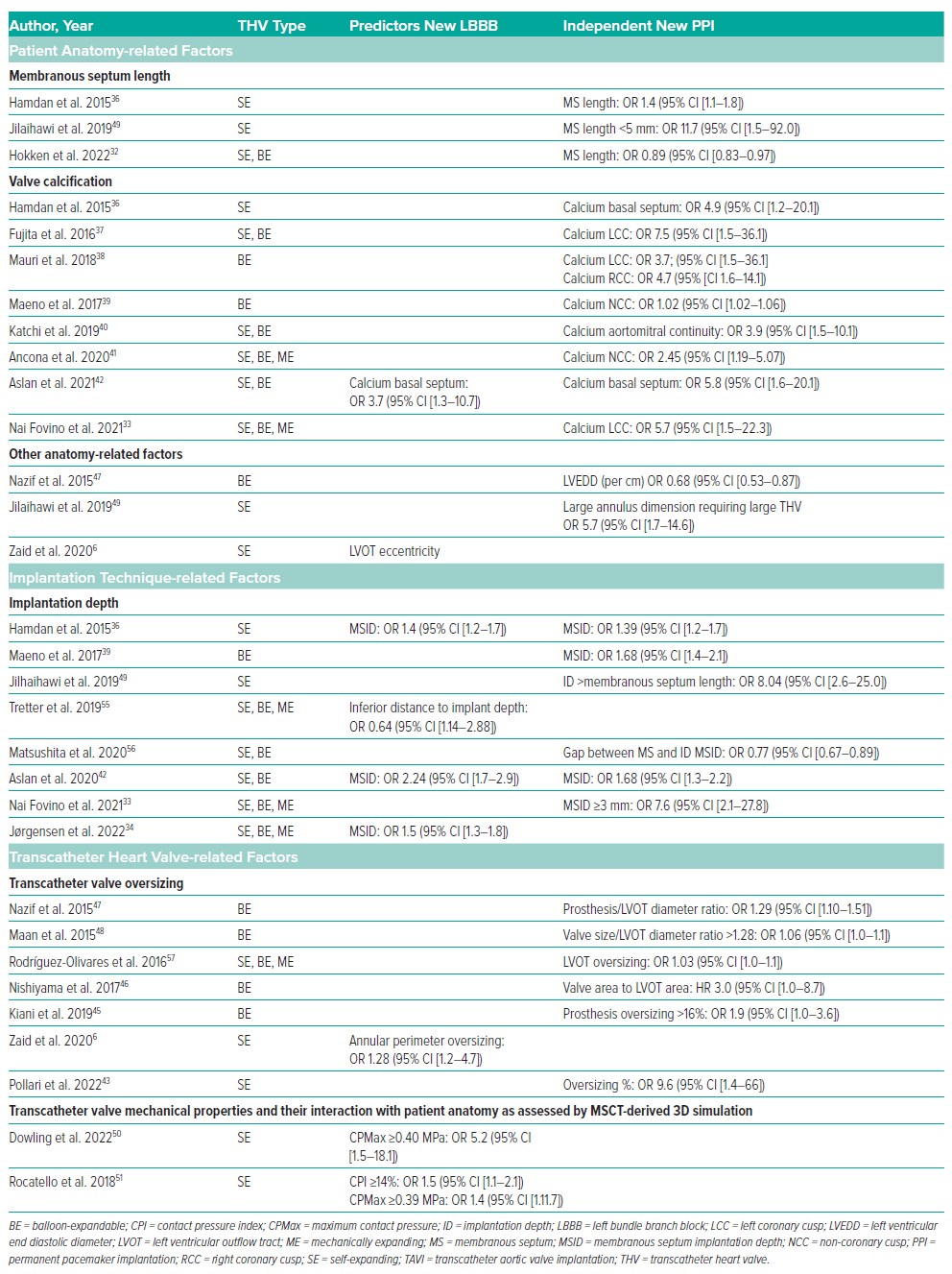
Beyond clinical and electrocardiographic predictors, an increasing number of studies demonstrate the value of MSCT data interpretation to predict and manage new LBBB and PPI after TAVI. MSCT offers detailed information on the dimensions, morphology and tissue characteristics (including calcium volume and distribution) of various aortic root structures near the conduction system. Using anatomical phenotypes and landmarks to estimate the location of the AV conduction system provides guidance to operators in the selection of THV type, size and ID with the goal of mitigating conduction disorders (Table 1). In addition, leveraging MSCT data for 3D computational simulations to predict the behaviour of the THV within the LVOT can help guide procedural strategy as outlined in Table 1.
Anatomy of the Conduction System
The aortic root is a tubular structure bordered by the ventricular septum in the LVOT and the sinotubular junction (STJ) in the aorta. It houses the three aortic valve leaflets that are supported by the sinuses of Valsalva and the interleaflet triangles interposed between the basal attachments of the leaflets. The interleaflet triangle between the right and non-coronary sinus is in direct continuity with the membranous part of the interventricular septum which contains the AV conduction bundle. In most patients the transition from membranous to muscular septum delineates the left ventricular location of the conduction system and, therefore, the membranous septum (MS) length may serve as anatomical proxy for the conduction system. Approximately 20% of patients have a different phenotype in which the AV bundle and conduction fibres run deep in the MS and seem less exposed at the LVOT surface.31
Membranous Septum Length and Implantation Depth
Recent research highlighted that patients with an MS (≤3 mm) exhibit a higher propensity for PPI (<20%) compared with patients with a long MS (>7 mm) regardless of ID and THV design (PPI >30%).32 The anatomical explanation is that individuals with a short MS exhibit the left ventricular part of the AV bundle in close proximity to the aortic annulus, increasing the likelihood of mechanical pressure trauma exerted by the THV. Correlating the MS length and ID further enhances risk prediction. Nai Fovino et al. found an 8-fold higher risk for pacemaker dependency when the overlap between MS length and ID was ≥3 mm.33 In a similar study Jørgenson et al. evaluated MS–ID overlap corrected for the MS morphology.34 The authors demonstrated that an overlap of ≥2.5 mm was the strongest predictor of CA when the MS length was measured at the anterior edge. Interestingly, the MS appeared 2.5 mm shorter at the anterior compared to the posterior edge, suggesting that measurement at the anterior edge closest to the right coronary cusp provides the most conservative MS length measurement.
In cases where significant MS–ID overlap seems unavoidable because of a short MS, consideration may be given to using the ACURATE neo THV which has low radial strength and a ‘top-down’ deployment mechanism to ensure a shallow ID with minimal LVOT interaction. Minimal MS–ID overlap (i.e. 1.0 mm) may cause CA during the initial phase, but a larger overlap of ≥3 mm is generally required to induce permanent CA.33 As such, in the context of minimal MS–ID overlap, an initial ‘wait and see’ approach after TAVI may save unnecessary pacemaker placements.
Calcification Patterns
Several studies investigated the relationship between the extent and distribution of calcium build-up in the aortic valvar complex and the occurrence of CA/PPI. Electrophysiological studies suggest that the degree of calcium deposition may reflect the severity of pre-existing conduction disease.35 Although total calcification burden was an important predictor of CA/PPI with older-generation THVs, these findings were not consistently reproduced in association with contemporary THV systems. Instead, the pattern or eccentricity of calcium depositions below the aortic valve appears more important.33,36–43 In a study involving self-expanding THVs, Hamdan et al. recently demonstrated that the combination of a high calcium burden below the LCC and low burden below NCC predicts PPI.36 An ex vivo simulation study showed that the THV is diverted away from the more calcified LCC area towards the area below the RCC/NCC commissure that harbours the conduction system, potentially causing pressure trauma and new CA/PPI.44 Other studies found a contradictory pattern among patients undergoing TAVI with balloon-expandable valves.39–41 The authors observed that patients with a high calcium burden below the NCC (not the LCC) predicts PPI (OR 1.02; 95% CI [1.02–1.06]). Similarly, excess calcification within the aortomitral continuity between the NCC and LCC (calcium score >300) was found to be associated with a 5-fold increased risk of pacemaker implantation.40 It is possible that the high radial force exerted by the stent frame of balloon-expanding devices may cause calcium deposits to compress directly against the underlying conduction tissue. It remains to be investigated how eccentric calcification patterns influence the pathophysiological mechanisms of conduction injury per THV technology.
Oversizing
A degree of prosthesis oversizing is essential to ensure safe anchoring of the prosthesis stent frame in the aortic annulus and minimise risk of paravalvular leakage, but it can lead to increased compression force on the conduction tissue. Self-expanding THVs typically exert less radial force but require more oversizing (~15%) compared to balloon-expandable THVs (~3%). An over-expansion of >20% of self-expanding THVs and >15% of balloon-expandable THVs at annular level predicts PPI and new LBBB, respectively.43,45 At LVOT level, a large prosthesis-to-LVOT ratio was associated with new PPI after balloon-expanding THV implantation across multiple studies.46–48
Other Anatomic Predictors
Various other anatomic characteristics have been linked to CA/PPI after TAVI. Zaid et al. demonstrated that LVOT eccentricity >35% independently predicts new LBBB after TAVI with self-expanding THVs.6 In a large balloon-expandable THV series, Nazif et al. demonstrated that a smaller LV end-diastolic diameter predicts PPI (for each 10 mm, OR 0.68; 95% CI [0.53–0.87]).47 Conversely, patients with large anatomies on MSCT who are scheduled to receive a large self-expanding THV (34-mm Evolut) seem also at risk for PPI.49
3D Simulations
MSCT-derived patient-specific computer simulation can predict the interaction between the THV and the native anatomy. The simulations provide unique insights on the behaviour of the THV (of any design/size) in individual patients at different IDs that can be used to evaluate the risk of paravalvular leakage and conduction abnormalities. The concept of the technology is that first, a 2D MSCT scan is converted into a 3D finite element model of the aortic root. Second, a digital THV (of any type/size) is incorporated into the model at various IDs. The simulations account for the geometric and mechanical properties of both the THV and surrounding tissue to predict stent-frame deformation and tissue compression. To assess the risk of new CA, a contact pressure analysis can be performed in the region where the AV bundle and left bundle branch surface in the LVOT. This region spans from the base of the NCC-RCC interleaflet triangle to the inferior edge of the MS. Data indicate that the percentage of this area (contact pressure index, CPI) and maximum contact pressure (CPMax) exerted by the THV are predictive of CA, with optimal cut-offs being CPI ≥14% and CPMax ≥0.40 MPa.50,51 A small observational study evaluated the clinical value of patient-specific computer simulation in 48 patients who were scheduled for TAVI using a self-expanding THV. The simulations did not affect valve size selection but did affect selection of the target ID.52 The ongoing multi-centre randomised controlled GUIDE TAVI trial will substantiate the true added clinical value of this innovative technology in a larger cohort of 454 patients.53 Finally, it has been proposed to integrate statistical (i.e. machine learning) and mechanical (i.e. patient-specific simulations) modelling to provide patient-specific estimation of CA risk after TAVI. Galli et al. demonstrated that a supervised machine-learning approach based on anatomical, procedural and mechanical data (derived from patient-specific simulations) achieved 83% accuracy (area under the curve 0.84) to predict new CA after TAVI (sensitivity 100%, specificity 62%, positive predictive value 76%, negative predictive value 100%, F1 score 82%).54
Conclusion
Novel transcatheter valve designs, refined implant techniques and advanced imaging tools may help mitigate the incidence of new conduction disorders and PPI after TAVI.










