Aorto-ostial intervention presents complex and unique challenges due to high calcium content, fibrous plaque and increasing elastic recoil related to the muscular elastic tissue. There are also technical problems, such as catheter engagement, poor guide catheter support and pressure damping. Additionally, ostial anatomic variations, the coronary ostium irregular shape and limitations of 2D angiography can lead to imprecisions in aorto-ostial stenting.1–3
Incomplete coverage of the ostial lesion or an excessive proximal stent protrusion is frequently encountered in aorto-ostial interventions. These occurrences are closely linked to adverse cardiovascular events, such as increased neo-intimal hyperplasia, incomplete neo-endothelisation, as well as early and late stent malapposition. The use of intracoronary imaging has been linked to improved clinical outcomes in ostial lesion stenting.2,3
Protruding stents that have been in place for a long time have not been extensively studied. Presented here is a case of a 10-year-old, 9.1 mm protruding stent in the right coronary artery aorto-ostial region that was associated with a positive exercise test in a patient with stable angina.
Case Presentation
A 54-year-old man with hypertension, diabetes and hyperlipidaemia presented with a positive exercise test. A decade earlier, he had extensive stenting of the right coronary artery (RCA) in the context of non-ST-segment elevation MI (NSTEMI), followed by staged procedures to left main (LM), left anterior descending (LAD) and left circumflex (LCx) arteries later in the same year. The patient was known to have normal left ventricular function. Ostial LCx required in-stent intervention 3 years later.
Current coronary angiography revealed widely patent bifurcation stents of the LM, LAD and LCx, moderate mid-LAD in-stent restenosis (ISR) and moderate native distal LAD disease. There was no significant ISR of ostial LCx or large obtuse marginal (OM) and severe focal stenosis of the distal OM. RCA stented from ostium to distal vessel showed mid-vessel and ostial ISR (Figure 1).
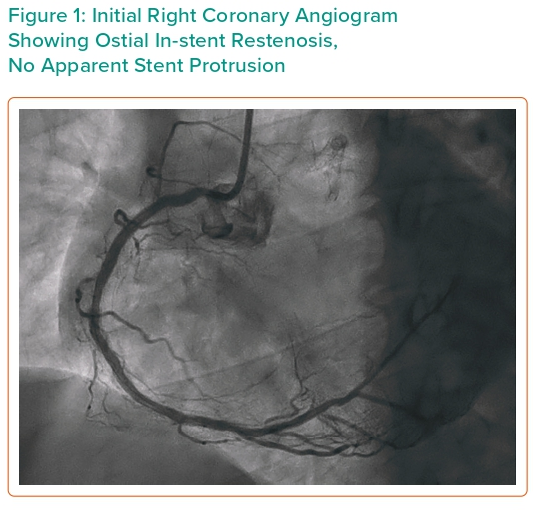
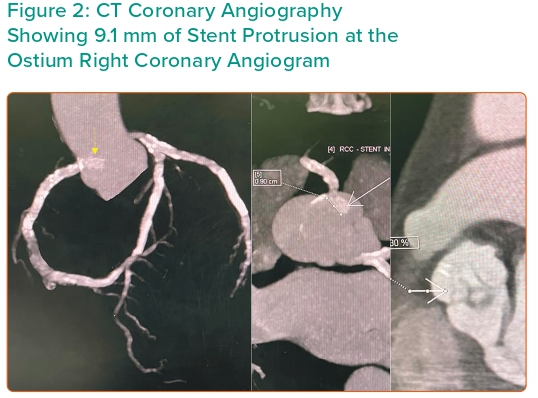
Using a 6 Fr Judkins right 4 (JR4) guide catheter with easy progression of wire, intravascular imaging revealed an area of 3.7 mm2 on the mid RCA. There was also pressure damping at the ostium suggestive of ostial lesion. We proceeded to percutaneous coronary intervention (PCI) to RCA ISR with 3.0/10 mm cutting balloon inflated in the mid, proximal and ostial lesions. At this moment we noticed that there was marked aortic protrusion of the 10-year-old stent. The wire, imaging catheter and balloon had crossed through the side of the stent without resistance. Discussion among the intervention team led to interruption of the procedure for fear of stent fracture with embolisation. CT coronary angiography (CTCA) demonstrated a 9.1 mm stent protrusion from the ostial RCA, not touching the aortic valve cusp (Figure 2).
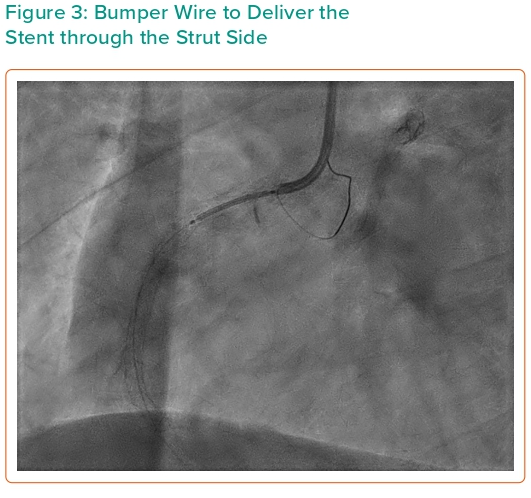
Three months later, the patient complained of occasional chest tightness and again had a positive treadmill test. He agreed to the procedure. There was pressure damping at ostial RCA with a 6 Fr JR4 guide catheter. Intravascular ultrasound (IVUS) was advanced over a floppy guide wire (ASAHI SION® blue) through the side strut of the old stent near the true ostium showing a minimum stent area (MSA) of 6.9 mm2, consisting mainly of fibrous plaque; proximal reference vessel size 4.5–5.0 mm. Following pre-dilatation with a 4.5/12 mm non-compliant (NC) balloon, a 4.5/15 mm drug-eluting stent (DES; Resolute Onyx) was deployed, covering the proximal vessel to the ostium. A bumper wire was placed to accurately position this stent. IVUS-guided optimisation with 4.5/8-mm NC balloon was used. The old stent remained in the same position.
Discussion
Aorto-ostial Lesion
A coronary aorto-ostial lesion (AOL), defined as a stenosis exceeding 50% within 3 mm from the coronary ostium, is reported to occur in approximately 0.18–2.7% of patients diagnosed with coronary artery disease (CAD).4
The prevalence of AOL varies depending on the population under study, with a higher incidence noted in the RCA. Among an acute MI cohort, AOL was found to occur in 45% of cases in the RCA and 8% in the left coronary. Additionally, RCA AOL was observed in 37% of cases of sudden cardiac death and 7% of instances of violent death.5 The predominance of RCA AOL may be explained by its higher elastic composition and nontubular anatomy.2
AOL are highly fibrotic, more densely calcified, and more prone to elastic recoil related to their muscular elastic tissue. Procedures are also more challenging and of higher risk due to adverse catheter engagement, poor guide support and frequent pressure damping. Concomitantly, anatomic variations of coronary origin, irregular ostial shape, and the limitation of 2D angiography can lead to imprecisions in ostial demarcation.1–3
Therefore, instances of incomplete coverage of the ostium or excessive proximal stent protrusion, collectively called ‘geographic miss’, are not infrequent and are closely linked to adverse cardiovascular events. Accurately performing coronary stenting in the aorto-ostial region can be challenging due variable take-off angles of the ostia of the left and right coronary arteries within the coronary sinuses. The ostial structures exhibit a funnel-like shape, with a larger diameter positioned at the proximal end. This complex 3D anatomy is often missed in 2D angiography. Additionally, there is risk of stent slippage at the moment of deployment. Following ostial stenting, observation of increased neo-intimal hyperplasia, incomplete endothelization, along with early and late stent malapposition is frequent.2–4
Stent Protrusion
Adequate deployment of a stent at the ostium of a coronary artery entails the fine balance between appropriate coverage and avoidance of overhanging. Existing practice recommends a maximum stent protrusion within the range of 1–2 mm into the aorta. Incomplete coverage, often referred to as a ‘geographic miss’, increases the likelihood of ISR, while excessive stent protrusion into the aorta can result in complications such as poor endothelisation with risk of stent thrombosis and, in extreme cases, trauma to the aortic valve cusps. Excessive stent overhang also poses challenges for subsequent interventions.6,7
The association between stent protrusion and stent thrombosis is explained by two mechanisms. First, protruding struts disrupt coronary blood flow, leading to increased shear stress at the stent edge and downstream, coupled with compromised coverage of the stent struts. This increased shear stress can induce platelet activation and subsequent thrombotic events. Second, incomplete strut coverage is linked to the development of late stent thrombosis. The impact of this mechanism becomes more significant as the gap between the stent struts and the vessel wall increases. Over time, stent strut coverage occurs, resulting in the formation of a neointimal bridge that disrupts blood flow. Neointima hyperplasia of these newly formed compartments progressively reduces lumen and flow.8
Avoiding Stent Protrusion
Reddy et al. demonstrated that the bumper wire or double wire technique is a secure and efficient approach for aorto-ostial interventions, with reduced geographic miss on IVUS and favourable clinical outcomes.3 Kerim et al. documented a case of acute stent thrombosis involving restenosis of a protruding ostial stent of the right coronary artery (RCA), with significant overhanging into the aorta, managed employing a double wire technique.1,2,6
When the protrusion is short, the proximal stent struts can be expanded outward against the aortic wall using a flash ostial balloon. Flaring of the stent may also be achieved by repeat balloon inflations downwards and upwards.2,6,9
Management of In-stent Restenosis in an Overhanging Stent
Re-intervention in the setting of a protruding stent is more complex due to challenges in catheter engagement and coaxial alignment, stent wiring through true lumen and high likelihood of deformation of the initial stent.4 Intervention through the side of a stent carries the potential risk of stent deformation or dislocation, and the likelihood of stent embolism. It may also prove impossible to advance devices due to steep angulation and rigid metallic obstacle.
In cases of excessive stent overhang, alternative techniques may be necessary, including double wire insertion, balloon-assisted procedures, snare devices, sequential ballooning of side struts and catheter extension-assisted side-strut stenting techniques.2
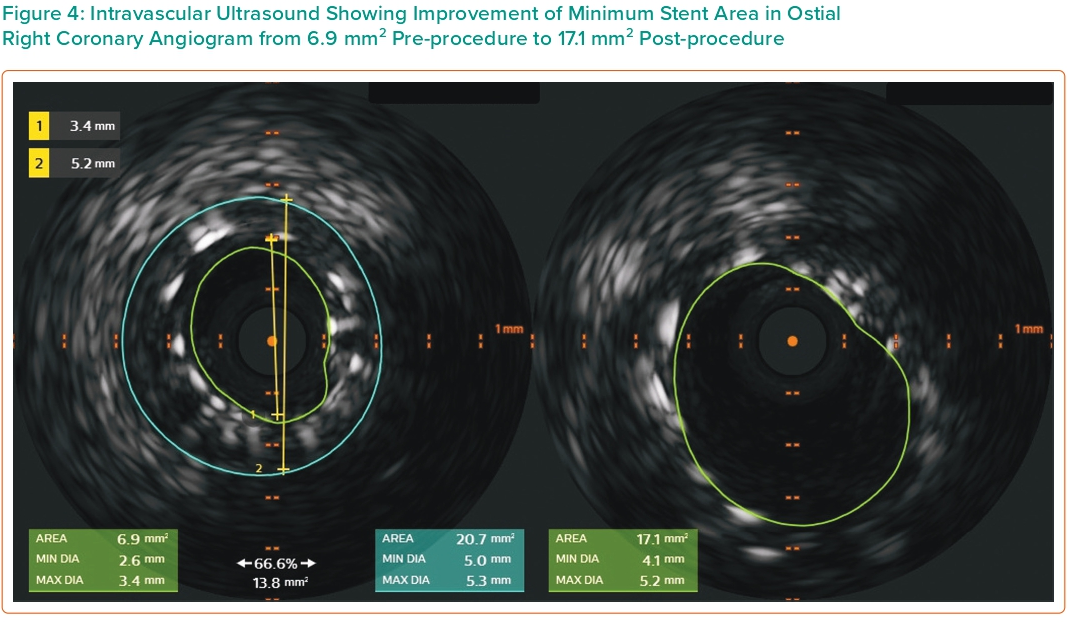
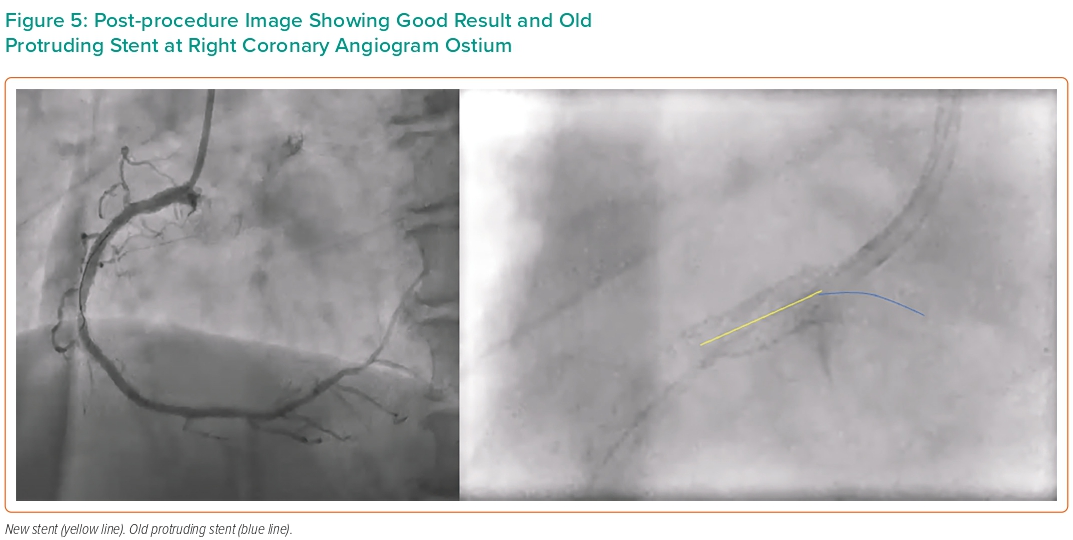
In the present case, the patient had a very favourable outcome over 10 years with no thrombosis. The mechanism of ISR is most likely in-stent hyperplasia, reflected by the fibrotic nature of plaque visualised on IVUS. The initial procedure entered the stent through the side and used a cutting balloon. On the second procedure, we employed the bumper wire technique for anatomical reference of the ostium thus avoiding geographic miss (Figure 3). The side-strut sequential balloon technique proved to be relatively straightforward and uncomplicated. This approach allows the guidewire to pass through the side-struts of the protruding stent, creating a new orifice through sequential ballooning. IVUS showed under-expansion and malapposition of the previous stent with an MSA of 6.9 mm2. Imaging post PCI confirmed good DES placement at the ostium and an increment of MSA to 17.1 mm2 (Figures 4 and 5).
Conclusion
Coronary aorto-ostial interventions require additional care to achieve accurate positioning of stent with good ostial coverage, and minimal stent protrusion (ideally 1–2 mm). Longer protrusion creates a nidus for thrombosis and can damage aortic valve leaflets. The present case is particularly unusual as the protrusion was 9.1 mm long and remained asymptomatic for 10 years. An initial intervention to in-stent restenosis caused deformation of the stent as was documented by CTCA. Successful intervention was achieved using an IVUS-guided, double wire technique with implantation of a second stent.