Antiplatelet Therapy
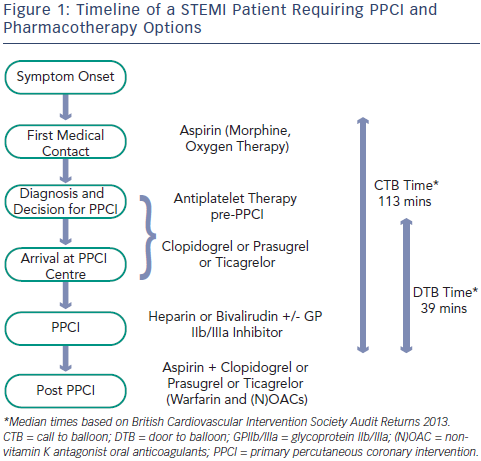
Current guidelines support the early administration of oral antiplatelet agents upstream of angiographic assessment and intervention.1 Aspirin is commonly given by the first medical contact and additional oral antiplatelet drugs are administered on arrival in hospital (see Figure 1).
Aspirin
The efficacy of aspirin in acute ST-segment elevation myocardial infarction (STEMI) was first demonstrated in the Second International Study of Infarct Survival (ISIS-2).2 In ISIS-2, 17,187 patients were randomised within 24 hours of an acute STEMI to receive oral aspirin 160 mg/day for 30 days, intravenous streptokinase, both agents or neither drug. Compared with placebo, aspirin therapy resulted in a highly significant reduction in vascular mortality (23 % odds reduction [OR]), equivalent to streptokinase monotherapy (25 % OR). The combination of aspirin and streptokinase offered even greater benefit (42 % OR). Aspirin therapy was also associated with significant reductions in the incidence of non-fatal re-infarction (1.0 versus 2.0 %) and stroke (0.3 versus 0.6 %) with no increase in the risk of major bleeding or haemorrhagic stroke.
Aspirin has excellent bioavailability and this is enhanced by use of uncoated aspirin, administered chewed or crushed to establish a high blood level quickly (time to peak concentration [Tmax] 20–30 minutes).3 Interestingly, there is a significant geographic variation in the dosing of aspirin. In Europe, the recommended oral loading dose is 150–300 mg (or intravenous [i.v.] 80–150 mg) followed by 75–100 mg by mouth (p.o.) daily.1 US STEMI guidelines recommend 162–325 mg loading followed by 81–325 mg daily.4
Clopidogrel
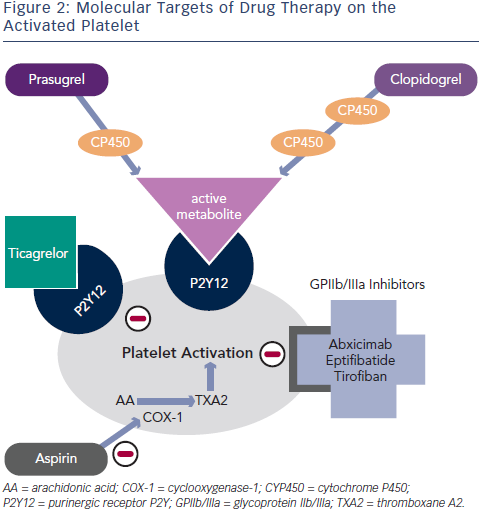
Clopidogrel is a thienopyridine – a pro-drug requiring two cytochromep450 dependent steps to generate an active metabolite – which binds irreversibly to the P2Y12 adenosine diphosphate (ADP) receptor on platelets (see Figure 2). Genetic polymorphisms in the cytochrome P450 (CYP) enzymes can lead to lower levels of the active clopidogrel metabolite, diminished platelet inhibition and a higher rate of major adverse cardiovascular events (MACE), including stent thrombosis. Approximately 30 % of healthy subjects have been shown to be carriers of a reduced function CYP2C19 allele.5
Use of clopidogrel in STEMI patients has evolved from initial trials in acute coronary syndrome (ACS) patients undergoing percutaneous coronary intervention (PCI) (Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial [PCI-CURE])6 and patients with STEMI treated with fibrinolysis before PCI (PCI-clopidogrel as adjunctive reperfusion therapy trial [PCI-CLARITY]).7 In PCI-CURE, ACS patients undergoing PCI benefited from combined treatment with clopidogrel and aspirin, achieving a 31 % reduction in cardiovascular death and MI at 30 days. In PCI-CLARITY, clopidogrel pre-treatment in STEMI patients undergoing fibrinolysis led to a 46 % reduction in the 30-day rate of cardiovascular death, recurrent MI or stroke compared with placebo, without an increase in bleeding.
The recommended clopidogrel loading dose in STEMI patients is 600 mg. Results from the Intracoronary stenting and antithrombotic regimen: Choose between 3 high oral doses for immediate clopidogrel effect (ISAR-CHOICE) trial8 showed that in patients undergoing PCI, loading with 600 mg of clopidogrel (compared with 300 mg) resulted in higher plasma concentrations of the active metabolite and lower values for ADP-induced platelet aggregation 4 hours after drug administration. The clinical benefit of a 600 mg loading dose in STEMI patients undergoing PPCI was demonstrated in the Antiplatelet therapy for reduction of myocardial damage during angioplasty (ARMYDA)-6 MI,9 Clopidogrel and aspirin optimal dose usage to reduce recurrent events – seventh organisation to assess strategies in ischemic symptoms (CURRENT-OASIS) 710 and Harmonising outcomes with revascularisation and stents in acute myocardial infarction (HORIZONS-AMI)11 trials. In ARYMDA-6 MI, high dose loading reduced infarct size with improved cardiac function, and 30-day MACE rates. Similarly, in subgroup analyses of the CURRENT-OASIS 7 and HORIZONS-AMI trials, STEMI patients loaded with clopidogrel 600 mg, prior to PPCI, had a significant reduction in stent thrombosis and myocardial infarction, without any increase in bleeding events.
Prasugrel
Prasugrel is a third-generation thienopyridine, sharing the same active metabolite as clopidogrel (see Figure 2), and despite partial reliance on CYP2C19, achieves faster and more potent platelet inhibition (a 60 mg loading dose of prasugrel reaches maximal plasma concentration at 30 minutes in healthy volunteers).12 Prasugrel has a very low rate of nonresponders in comparison with clopidogrel.13
The clinical superiority of prasugrel over clopidogrel in ACS was demonstrated in the TRial to assess Improvement in Therapeutic Outcomes by optimising platelet inhibitioN – Thrombolysis in Myocardial Infarction-38 (TRITON–TIMI 38) study.14 Prasugrel, administered following angiography, reduced the composite primary endpoint (cardiovascular death, non-fatal MI or stroke) in patients undergoing PCI for STEMI or moderate-high risk ACS. In the pre-specified STEMI subgroup (3,534 patients), the risk reduction was 21 % (prasugrel 10 % versus clopidogrel 12.4 %) at 15 months, without a significant increase in non-coronary artery bypass graft (CABG)-related bleeding.15 The risk of stent thrombosis was also significantly lower.
Prasugrel is contraindicated in patients with prior stroke/transient ischaemic attack (TIA), and is not recommended in patients aged ≥75 years or in patients with lower body weight (<60 kg), as there was no net clinical benefit in these subsets. A reduced maintenance dose of 5 mg could be considered in these patients.
Ticagrelor
A new chemical class called CycloPentylTriazoloPyrimidine is partly formed by Ticagrelor, which, in contrast to thienopyridines, causes reversible inhibition of the P2Y12 receptor and does not require hepatic metabolism for its activity (see Figure 2).16 Similar to prasugrel, ticagrelor provides more rapid, potent and consistent platelet inhibition over clopidogrel.
In the PLATelet inhibition and patient Outcomes (PLATO) trial,17 ticagrelor (compared with clopidogrel) reduced the composite primary endpoint (cardiovascular death, non-fatal MI or stroke) and also reduced cardiovascular mortality in STEMI and moderate–high risk ACS patients. In the STEMI subgroup, this primary endpoint was reduced from 10.8 % in the clopidogrel group to 9.4 % in the ticagrelor group (relative risk [RR] reduction of 13 %). In addition, overall mortality was reduced from 6 % to 4.9 % without a higher risk of major bleeding.
Dyspnoea is a frequently reported side effect of ticagrelor. In PLATO, 13.8 % of patients on ticagrelor reported dyspnoea compared with 7.8 % treated with clopidogrel.17 However few patients (0.9 %) discontinued the drug because of dyspnoea; importantly, there were no associated lung abnormalities and the mortality benefit persisted in this group.18 Contrary to the PLATO experience, a recent study of ticagrelor compliance in ACS patients demonstrated that dyspnoea was the commonest reason for drug discontinuation, occurring in 9.1 % of cases.1
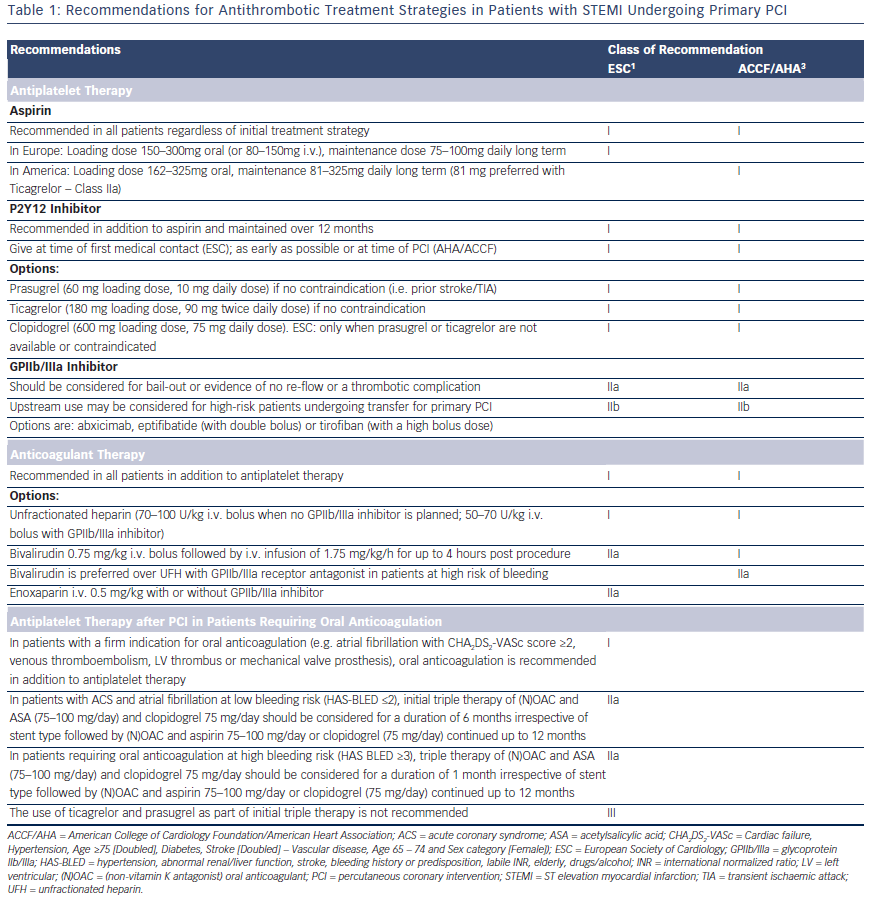
The European Society of Cardiology (ESC) and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) recommendations for antithrombotic strategies in patients with STEMI undergoing primary PCI are summarised in Table 1. Prasugrel, ticagrelor and clopidogrel (600 mg loading dose) are all class I, level B options in both guidelines, but the ESC expresses a clear preference for the newer antiplatelet agents, stating that clopidogrel should only be used when prasugrel or ticagrelor are either not available or contraindicated.
Glycoprotein IIb/IIIa Inhibitors
Glycoprotein IIb/IIIa inhibitors (GPIs) provide rapid, potent platelet inhibition. Their use in PPCI has spanned the evolution of PCI and pharmacological therapies; consequently, it is challenging to relate the data to current practice with more potent oral antiplatelet therapies. Initial data supported the combined role of stenting and abciximab administration to minimise target vessel revascularisation,20 and preangiographic commencement of therapy appeared advantageous.21 However, in the dual antiplatelet therapy (DAPT) era, early use of abciximab resulted in an increased rate of bleeding.22 Subsequent analysis has demonstrated a continued benefit in early administration of GPIs in high-risk patients,23 particularly if presenting early or to a non-interventional centre.24 Contemporary trials provide conflicting results, the ONgoing Tirofiban in Myocardial infarction Evaluation 2 (ON-TIME 2) trial,25 utilising pre-hospital initiation of high bolus dose tirofiban, in addition to aspirin, heparin and high-dose clopidogrel, reduced MACE at 30 days with no significant increase in major bleeding. However, the HORIZONS-AMI26 trial demonstrated superiority of bivalirudin versus unfractionated heparin (UFH) and GPI in terms of a composite of major bleeding and MACE. Consequently, current guidelines1,4 suggest restricting GPI use for ‘bailout’ in the event of angiographic evidence of massive thrombus, slow-/no-reflow or a thrombotic complication.
Adjunctive Antithrombotic Pharmacotherapy during PPCI
In addition to the array of oral antiplatelet therapy options in PPCI, there is continued debate regarding the optimal combination of antithombotic therapy. UFH and bivalirudin are most commonly used and the European guidelines for STEMI support their use with a Class I indication.27 The evidence supporting use of bivalirudin derived from HORIZONSAMI, 26 which demonstrated that bivalirudin compared with UFH and routine use of GPIs was associated with a reduction in mortality and major bleeding at 30 days, with a survival benefit that extended to 3 years. Further support for bivalirudin’s bleeding safety was demonstrated in the open-label European Ambulance ACS Angiography (EUROMAX) trial,28 comparing pre-hospital administration of bivalirudin versus UFH or low-molecular-weight heparin (LMWH) with optional use of GPI (58.5 % routine use). However, both trials were associated with an increased rate of AST with the use of bivalirudin and the elevated bleeding rate observed in the UFH arm of both studies has been attributed to the high rates of GPI use.
The recently published How Effective are Antithrombotic Therapies in primary percutaneous coronary intervention (HEAT-PPCI) trial29 was designed to specifically address the criticisms levelled at previous bivalirudin trials, specifically the efficacy of bivalirudin monotherapy against UFH with GPI use restricted to true ‘bail-out’ (13 % and 15 %, respectively). The study demonstrated a primary efficacy outcome (allcause mortality, cerebrovascular accident, re-infarction or unplanned target lesion revascularisation) of 8.7 % in the bivalirudin group versus 5.7 % in the heparin group (RR 1.52, 95 % confidence interval [CI] 0.9– 2.13). This reduction in major adverse ischaemic events with heparin was not associated with an increase in bleeding complications. Definite or probable stent thrombosis occurred more often with bivalirudin (3.4 versus 0.9 %, RR 3.91, 95 % CI 1.61–9.52). In the light of these new data, the most recent ESC guidelines on revascularisation have downgraded their recommendation for the use of bivalirudin to Class IIa1.
Acute Stent Thrombosis
The increased rate of AST observed with bivalirudin therapy has been attributed to the relatively short half life of bivalirudin (t1/2=25 minutes),30 resulting in a waning effect of the drug within 2 hours of withdrawal. The risk of AST is further exacerbated by the observed delay in platelet inhibition observed in STEMI patients treated with oral P2Y12 inhibitors. The Rapid Activity of Platelet Inhibitor Drugs (RAPID) Primary PCI study31 evaluated 50 patients with STEMI undergoing PPCI with bivalirudin monotherapy, randomised to prasugrel or ticagrelor at standard loading doses. There was no significant difference in residual platelet reactivity between both drugs but the study showed that effective platelet inhibition within 2 hours of loading was only achieved in half of patients. Four hours were required to achieve effective platelet inhibition in the majority of patients.
Morphine Effect on Platelet Activity
The RAPID investigators assessed the effect of opiate use on platelet reactivity. The use of morphine significantly affected the activity of prasugrel and ticagrelor, independently predicting high residual platelet reactivity 2 hours post-loading dose (odds ratio 5.29; p=0.012).31 The effect has been confirmed in a randomised controlled trial of 24 healthy subjects receiving 600 mg of clopidogrel with placebo or 5 mg of intravenous morphine. Morphine was shown to delay clopidogrel absorption, decrease plasma levels of the active metabolite and delayed the maximal inhibition of platelet aggregation by 2 hours.32 Furthermore, the opiate effect on platelet inhibition does not appear restricted to patients experiencing opiate-related nausea/vomiting.33
The negative interaction between morphine and oral antiplatelet agents is also supported by the Administration of ticagrelor in the cathlab or in the ambulance for new STEMI to open the coronary artery (ATLANTIC) trial,34 which demonstrated that the primary end point of ST-segment resolution was significantly improved with prehospital administration of ticagrelor in opiate-naïve patients. These findings challenge current guidance to administer analgesia early, on first medical contact.27
Methods to Enhance Platelet Inhibition
Studies to overcome the potential delay in platelet inhibition, associated with immediate pre-procedural loading of oral antiplatelet therapy, have been undertaken. The ATLANTIC investigators addressed this question by randomising 1,862 patients presenting within 6 hours of STEMI onset to pre-hospital versus in-hospital treatment with ticagrelor.34 Pre-hospital ticagrelor did not improve pre-PCI coronary perfusion but appeared to be safe and was associated with a reduction in post-procedural AST. The median time between the two loading doses (pre-hospital versus in-hospital) was 31 minutes.
Alternatives to upstream administration of an oral antiplatelet therapy include manipulation of the pharmacokinetic properties of oral agents or use of an intravenous platelet inhibitor. The Mashed Or Just Integral Tablets of ticagrelOr (MOJITO) study35 tested the effect of crushing ticagrelor to accelerate drug absorption and demonstrated a significant enhancement of platelet inhibition 1 hour following drug ingestion. A larger scale trial with clinical endpoints would be necessary to validate these results. Cangrelor is an intravenous adenosine triphosphate (ATP) analogue, which reversibly inhibits the P2Y12 receptor without requiring hepatic conversion.36 The attraction of cangrelor is therefore its very rapid onset of action and short halflife (3–5 minutes) allowing rapid platelet inhibition and quick reversal. Two early trials (Cangrelor versus standard therapy to achieve optimal management of platelet inhibition [CHAMPION]-PCI37 and CHAMPIONPLATFORM38) evaluating cangrelor in patients undergoing PCI failed to show clinical superiority over clopidogrel. The more recent CHAMPION-PHOENIX39 trial randomised 11,145 patients undergoing urgent or elective PCI to intravenous cangrelor or clopidogrel 600 or 300 mg loading (56 % stable angina/18 % STEMI). At 48 hours the rate of composite primary efficacy endpoint (death, MI, ischaemiadriven revascularisation or stent thrombosis) occurred less in the cangrelor group (4.7 % versus 5.9 %) and the rate of stent thrombosis was significantly lower (0.8 % cangrelor versus 1.4 % clopidogrel). Although the study demonstrated benefit with use of cangrelor, there were significant limitations in the trial design, favouring the study drug arm. The control group only received clopidogrel once the anatomy was delineated, and 30 % of the cohort were administered the drug post-PCI. Consequently, the higher rate of peri-procedural MI in the control group is not surprising.
Antiplatelet Strategies in Patients on Oral Anticoagulation
A significant proportion of patients undergoing PPCI may already be anticoagulated on a vitamin K antagonist (VKA) or a non-vitamin K antagonist oral anticoagulants ((N)OAC) at the time of the procedure. These patients are at increased risk of bleeding and often the international normalized ratio (INR) levels are not available. There is no clear evidence on the optimal antithrombotic pharmacotherapy for these patients. The 2014 ESC/EACTS guidelines on myocardial revascularisation1 recommends that PPCI in this setting should be performed via a radial approach with use of additional parenteral anticoagulation regardless of the timing of the last dose of oral anticoagulant. Bivalirudin may be preferred due to its short half-life and should be discontinued immediately after PPCI. GPIs should generally be avoided unless for bail-out situations.
Duration of Dual Antiplatelet Therapy post-PPCI DAPT (aspirin + a P2Y12 inhibitor) is recommended for 1 year in patients undergoing PPCI for STEMI. This recommendation is based on the early CURE study40 (clopidogrel) and is supported by more recent results from TRITON-TIMI 38 (Prasugrel)15 and PLATO (Ticagrelor).17 Regardless of stent type, extended DAPT for 1 year reduces the risk of stent thrombosis, re-infarction and cardiovascular mortality6 with the more potent DAPTs associated with the greatest post-ACS clinical benefit.41 Recent data have highlighted that extended DAPT confers further protection against ischaemic events but at the expense of additional bleeding risk.42 In stark contrast, the Global-Leaders trial (NCT01813435) is currently enrolling patients to either 1 month DAPT with aspirin and ticagrelor, and then ticagrelor monotherapy for 23 month or 12 months DAPT with aspirin and ticagrelor/clopidogrel with aspirin monotherapy between 12 and 24 months. We await the results with interest.
Antiplatelet Therapy after PCI in Patients Requiring Oral Anticoagulation
A proportion of patients on DAPT post-PPCI will have a firm indication for long-term anticoagulation (atrial fibrillation with Cardiac failure, Hypertension, Age ≥75 [Doubled], Diabetes, Stroke [Doubled] – Vascular disease, Age 65 – 74 and Sex category [Female]) [CHA2DS2- VASc] score ≥2, venous thromboembolism, left ventricular [LV] thrombus, mechanical valve prosthesis). Triple therapy with an oral anticoagulant, aspirin 75–100 mg and clopidogrel 75 mg should be limited in duration depending on the clinical setting, thromboembolic risk (CHA2DS2-VASc score) and bleeding risk (HAS-BLED score). The WOEST trial43, which randomised 573 patients either to dual therapy or triple therapy, showed that in patients on oral anticoagulants the use of clopidogrel without aspirin was safe. TIMI bleeding and allcause mortality was lower in the dual therapy group with no increase in the rate of thrombotic events. The 2014 ESC/EACTS guidelines on myocardial revascularisation1 recommends 1 month of triple therapy for ACS patients at high bleeding risk (HAS-BLED >3) and 6 months for patients at lower bleeding risk, followed by dual therapy (oral anticoagulant and clopidogrel or aspirin) for a minimum of 12 months (see Table 1). The use of prasugrel and ticagrelor as part of triple therapy should be avoided44 and gastric protection with a proton pump inhibitor should be implemented.
Conclusion
Successful revascularisation of patients presenting with STEMI requires rapid transfer to a PCI capable unit and concomitant treatment with antiplatelet and antithrombotic drugs. A delicate balance exists between thrombosis and bleeding and a perfect combination of agents is yet to be found. An intimate relationship between the intravenous antithrombotic and oral antiplatelet agents exists and these must be considered in the selection/tailoring of treatment. Oral antiplatelet therapies provide long-term platelet inhibition but are hampered by delayed onset of action in acutely unwell patients. Future strategies may include upstream administration of drugs by the first medical contact or acute treatment with an intravenous platelet inhibitor.