Significant left main coronary artery (LMCA) disease is observed in 4–5% of patients undergoing coronary angiographies.1 Major adverse cardiac events (MACE) are more common because LMCA disease substantially affects myocardial supply. Until recently, coronary artery bypass surgery (CABG) has been the standard of care for most patients with LMCA disease because of its established mortality benefit over medical therapy, while percutaneous coronary intervention (PCI) was considered only as a salvage treatment.2
Technological advancements in stents, imaging and adjunctive pharmacotherapy have improved the outcomes of PCI for the management of LMCA disease. Data from large clinical trials (SYNTAX, PRECOMBAT, EXCEL and NOBLE), registries and meta-analyses support emerging evidence for the use of PCI for the management of LMCA disease.3–8 According to the updated American College of Cardiology/American Heart Association guidelines, PCI is recommended for LMCA disease in cases where it can provide revascularisation on a par with CABG (class 2a).9 The European Society of Cardiology recommendations for the use of PCI for LMCA disease are based on the anatomical complexity (SYNTAX score).10 This review focuses on the most recent published data on left main PCI, with a particular emphasis on the imaging component, current clinical trials and PCI guidelines.
Coronary Artery Bypass Surgery Versus Percutaneous Coronary Intervention Studies
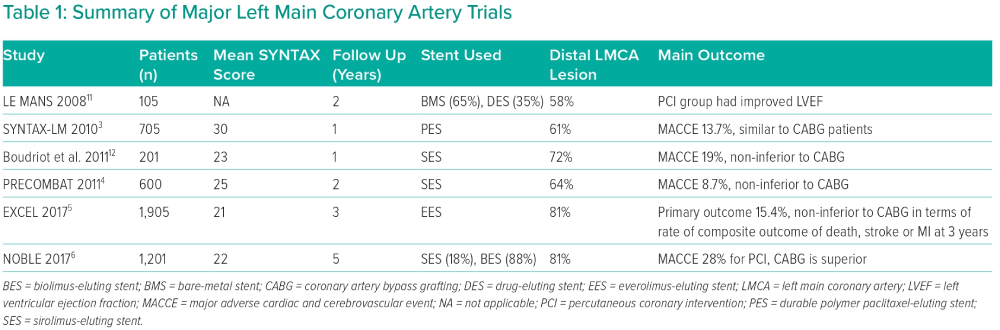
Six landmark trials have compared CABG with PCI for the management of LMCA disease (Table 1). The pioneering trials (LE MANS, SYNTAX, Boudriot et al. and PRECOMBAT) used first-generation drug-eluting stents (DES) and reported similar rates of death, MI and stroke for both strategies.3,4,11,12 Subsequently, two large trials (EXCEL and NOBLE) that used second-generation DES had conflicting results.5,6
A meta-analysis of five randomised trials including 4,612 unprotected LMCA (ULMCA) patients with a weighted mean follow-up duration of 67 months concluded that there were no significant differences in cardiac death, stroke or MI between PCI and CABG.11 Another recent meta-analysis of four randomised trials (PRECOMBAT, SYNTAX, EXCEL and NOBLE), with 4,394 patients and a mean SYNTAX score of 28, demonstrated no significant difference in 5-year all-cause death between CABG and PCI. However, spontaneous MI and repeat revascularisation were more common with PCI than CABG (p<0.0001). Mortality data from the SYNTAX and PRECOMBAT trials revealed no difference at 10 years (HR 0.96; 95% CI [0.76–1.21]).8 Thus, literature evidence suggests that the outcomes of mortality, infarction and stroke after 5 years do not differ between the cohorts treated with CABG and PCI.
The need for tailored patient selection and improvements in PCI technique is reinforced by these novel results. The importance of the multidisciplinary heart team is emphasised while making treatment decisions for stable or stabilised patients with ULMCA disease. A brief management flowchart of patients with ULMCA lesions is shown in Figure 1.
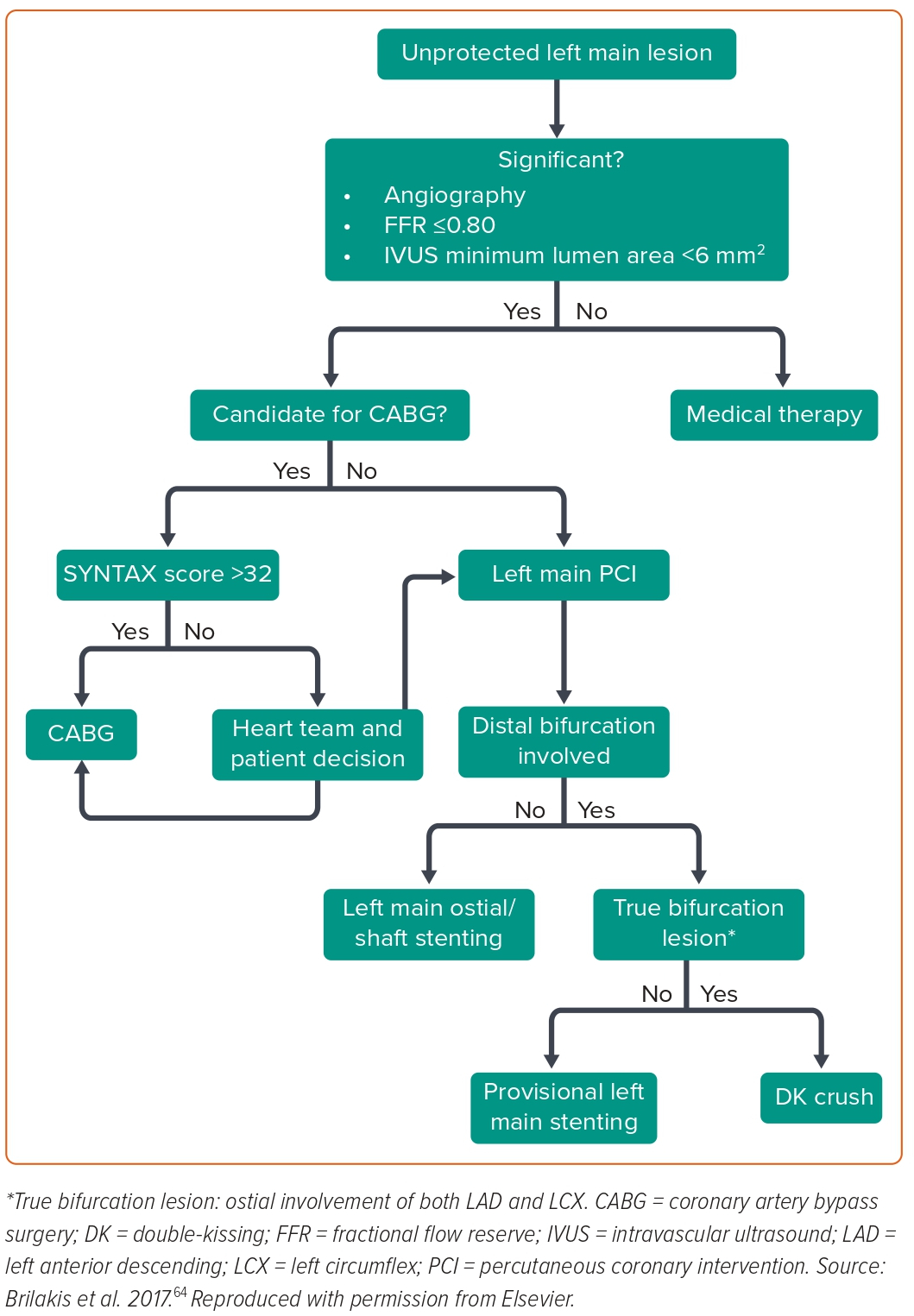
Technical Considerations with Left Main Coronary Artery Percutaneous Coronary Intervention
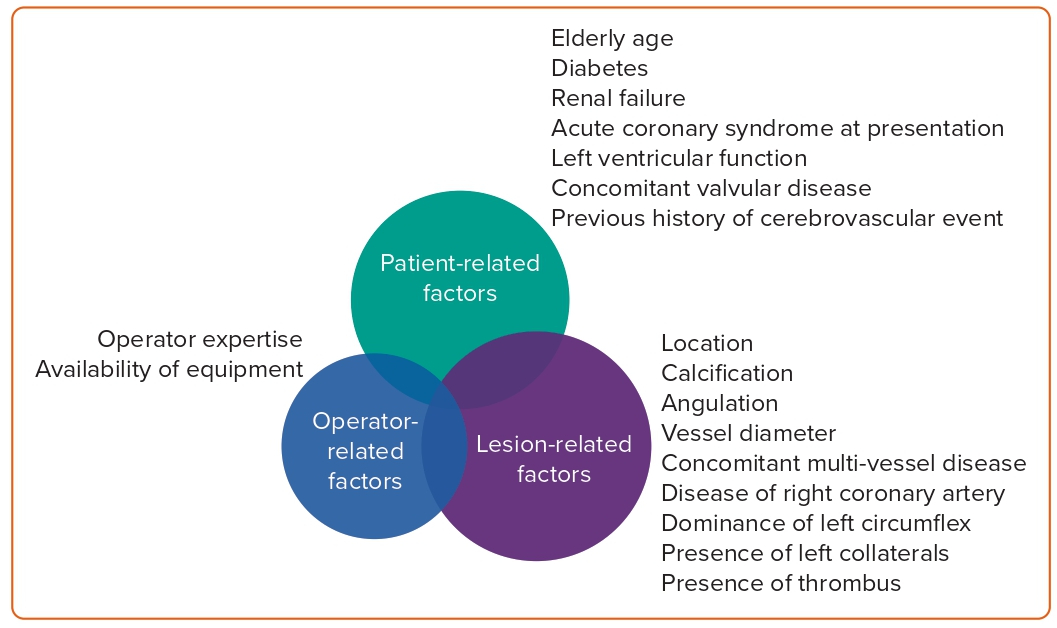
Patient-related Factors
Patient-related factors to consider include elderly age, diabetes, renal failure, acute coronary syndrome (ACS) at presentation, left ventricular function, concomitant valvular disease and previous history of cerebrovascular event.
Lesion-related Factors
Lesion-related factors to consider include location (ostial, shaft or distal bifurcation), calcification, angulation, vessel diameter, concomitant multivessel disease, disease of right coronary artery, dominance of left circumflex (LCX), presence of left collaterals and presence of thrombus. Lesion complexity may be assessed using the SYNTAX score.13
Operator-related Factors
Operator expertise is critical for the success of PCI procedures. The availability of appropriate equipment (mechanical circulatory devices, imaging, plaque modification hardware) also influences PCI outcomes. Evidence demonstrates that patients treated at large-volume facilities that frequently carry out these procedures have a better prognosis.14 These factors are highlighted in Figure 2.
Physiological Assessment of Left Main Lesions
The determination of fractional flow reserve is a crucial step in the decision-making process for LMCA intermediate stenosis. It is safe to delay revascularisation if the fractional flow reserve (FFR) score is >0.8.15 A meta-analysis of six trials on FFR in LMCA revealed that there was no difference between patients who were deferred based on FFR and those who had revascularisation in terms of the risk of the composite end goal of mortality, MI and future revascularisations. However, the deferred group had a greater rate of later revascularisation alone.16 No difference was observed in the rates of all-cause mortality or non-fatal MI.
A recent study demonstrated that employing the instantaneous wave free ratio (iFR) to delay revascularisation of LMCA is safe.17 However, non-hyperaemic pressure wire markers are not yet validated in LMCA disease. There is also an on-going trial to evaluate the concordance between FFR and iFR for the assessment of intermediate lesions in the LMCA (NCT03767621). The study results might shed further light on the use of iFR and its pathological threshold in the management of LMCA lesions.
Pressures must be equalised and measured using a guide catheter that has been partially engaged in the LMCA to prevent a presumed ostial lesion from affecting the measurement. It is always a better option to measure FFR by placing the pressure wire in both branches of the LMCA. For logical reasons, it is better to give an IV adenosine infusion than intra-coronary adenosine, as the guide catheter needs to be disengaged for accurate FFR measurement in LMCA disease.
It is important to note that the physiological interdependence of the coronary tree may alter the values of FFR. FFR is overestimated in cases of diffuse disease involving the left anterior descending (LAD) and LCX arteries. A pullback FFR measurement of the LMCA is appropriate to determine whether revascularisation is required in the context of distal vessel disease. In these situations, the use of iFR ‘scout’ pullbacks may also be advantageous because they make it simpler to physiologically map out individual parts of serial stenoses.18 FFR/iFR may also be used to examine the jailed LAD and LCX ostial lesions post-stenting.
Imaging in Left Main Disease
Imaging of LMCA is mandatory for the assessment of lesion morphology and optimisation post-PCI. The vessel size and plaque distribution within the LMCA and its daughter branches are characterised using intravascular ultrasound (IVUS). It allows precise minimum lumen area (MLA) measurements at the cross-sectional level. A prospective study demonstrated that safe deferral of LMCA revascularisation is possible in candidates with IVUS-derived MLA of ≥6 mm2.19 Another study showed correlation between MLA diameter (≤4.5 mm2) and functional significance in patients with isolated intermediate LMCA stenosis.20 The calcific burden in the LMCA can be determined with IVUS; this information can be used to determine upfront plaque-modification strategies and to assess post-stenting complications, such as edge dissection and stent deformation.
Optimisation is crucial after LMCA stenting. According to Kang et al., the optimal post-stenting minimal stent area (MSA) should be 8 mm2 in the LMCA and 7 mm2 at the level of the point of convergence (POC). This has been linked to increased survival.21 However, the outcomes of subsequent investigations vary in terms of LMCA MSA. Further insight into the achievable IVUS MSA was provided by a post hoc analysis of the IVUS core laboratory data from the NOBLE trial. The MSA criteria of LMCA (8 mm2), POC (7 mm2), LAD ostium (6 mm2) and LCX ostium (5 mm2) was not achieved in 3.6%, 0.5%, 5.2% and 7.6% of patients, respectively. When this ‘failure to achieve MSA’ group was compared to those who achieved satisfactory MSA, there were numerically higher numbers of event rates in the former group: major adverse cardiac and cerebrovascular events 36.8% versus 20%, death 15.8% versus 5.4% and repeat revascularisation 15.8% versus 11.7% respectively.22 None of these were statistically significant due to few event rates and a decreased number of patients who underwent core lab analysis. In the EXCEL trial, greater MACE rates (18.2%) were reported in patients who did not achieve post-PCI MSA of 9.8 mm2 in comparison to those who did (11.5%) at 3-year followup.23
Optical coherence tomography (OCT) evaluation of non-ostial left main (LM) is gaining importance because of the higher resolution of OCT compared with IVUS. OCT is more effective in detecting thrombus, dissection, extent of calcification and incomplete apposition of stent struts.24–26 The LEMON study is the first to assess the role of OCT in LM PCI according to a standardised protocol.27 This study included 70 patients with non-ostial LM stenosis from 10 centres in France. The pre-specified protocol consisted of three OCT runs. The first one was performed prior to stent implantation to evaluate plaque features, landing zones, lesion length, dimensions of the reference segment and stent and balloon diameter for proximal optimisation technique (POT). After stent placement, POT and side branch (SB) rewiring, the second run was carried out with the goal of assessing the re-cross wire in the jailed SB. After PCI optimisation, a third run was conducted to analyse stent expansion and identify significant strut mal-apposition and edge dissection. Further PCI optimisation was carried out in 26% instances following the second and third runs, indicating that OCT guidance altered the PCI approach in a quarter of patients.
To overcome the inherent difficulties in stent expansion assessment within bifurcated lesions, a novel OCT criterion called the ‘LEMON criteria’ was established. The stent was divided into two sections, using the carina as the cut-off point. The MSA was then measured in the proximal (upstream carina) and distal (downstream carina) sections. The ratio between MSA and reference MLA was calculated for both sections. The expansion was considered successful if the MSA/reference MLA was ≥80% in both proximal and distal stent sections. The primary endpoint of procedural success was a combination of residual stenosis of <50% by quantitative coronary angiography and Thrombolysis in MI 3 flow in all arteries. Adequate stent expansion was achieved in 86% of the cases. The MACE rate at 1 year was 1.4%. The wire position in the SB could be analysed in 98% of the patients. In 15% of the cases, the operators decided to reposition the wire based on OCT analysis.
Interestingly, despite the data supporting imaging in LMCA, imaging is used less frequently in clinical practice. The use of imaging was only around 40% even in major multicentre trials evaluating various stenting techniques for LMCA, such as DKCRUSH-V and EBC MAIN.28,29 Results from the ROLEX registry shows lower 1-year target-lesion failure (TLF; 2%) in patients who underwent intravascular imaging compared to angiography guided PCI (7.6%).30
Radial Versus Femoral Access for Left Main Percutaneous Coronary Intervention
Radial access is considered the default strategy for PCI in most centres around the world. LMCA PCI frequently requires a larger guiding catheter, use of intravascular imaging and large stents. Therefore, LMCA PCI is commonly performed via transfemoral access for better guide support, ease of procedure and anticipation of complexities. However, with newer devices (mother and child catheters, low profile radial sheaths and sheathless guiding catheters) and improved technical skills of operators, more LMCA PCIs are performed via transradial access (TRA) in few centres.
Currently, there are no randomised trials comparing the access site for LMCA PCI. A meta-analysis of eight retrospective studies involving 2,858 patients undergoing LMCA PCI showed reduced access site complications (OR 0.17; 95% CI [0.07–0.41]; I2 = 16%), major bleeding (OR 0.39; 95% CI [0.17–0.86]; I2 = 0%) and all-cause mortality (OR 0.28; 95% CI [0.12–0.64]; I2 = 0%) in the TRA group. There were no significant differences in in-hospital and long-term cardiovascular mortality, MI and MACE between the two groups.31 Another systematic review of 12 observational studies with 17,258 patients also showed TRA was associated with a significant reduction in access site bleeding (OR 0.11; 95% CI [0.04–0.26]; p<0.0001), major bleeding (OR 0.44; 95% CI [0.27–0.69]; p=0.0005) or any bleeding episode (OR 0.43; 95% CI [0.27–0.69]; p=0.0004). Rates of access site or vascular complications (OR 0.26; 95% CI [0.17–0.40]; p<0.00001) and in-hospital mortality (OR 0.49; 95% CI [0.31–0.79]; p=0.004) were also lower in the TRA group. Interestingly, there was also a lower rate of long-term target vessel revascularisation in the TRA group (OR 0.62; 95% CI [0.41–0.94]; p=0.020).32
Only 27% of the participants in the SYNTAX trial received TRA. However, in recent major LMCA trials, such as DKCRUSH-V and EBC-MAIN, TRA usage was approximately 70–75% and 77% in the ROLEX registry.
Strategies for Left Main Percutaneous Coronary Intervention
Left Main Ostial and Shaft Stenting
Ostial and shaft lesion PCI can be easily carried out using an appropriately sized single stent and optimising the result with post-dilation using a non-compliant balloon with the help of IVUS/OCT. Non bifurcation lesions (ostial and mid-shaft) show improved outcomes with PCI in comparison to bifurcation lesions because of their large lumen dimensions and decreased probability of plaque displacement and restenosis.33–35 Hence, ostial, mid-shaft stenting is the ideal scenario for ULMCA PCI. Long term outcomes are superior to CABG. However, only approximately 30% of lesions occur in this region.36
When treating ostial LMCA lesions, cranial views (left anterior oblique, anteroposterior and right anterior oblique) are preferred to allow adequate visualisation of the ostium and ensure adequate protection.37 The use of side holes in guide catheters has been traditionally preferred for pressure damping and ventricularisation.38 Instead, the standard guide that has the guidewire loaded on should be used. The guide may be safely and quickly withdrawn after the passage of the guidewire. Side holes also increase the contrast volume and weaken the tip of the guide catheter.39 Hence, the lack of side holes allows more accurate stent positioning with minimal protrusion. Following stent deployment, adjunctive balloon post-dilatation is needed to optimise the stent and to to allow for easy recannulation if a repeat coronary angiography or PCI is performed in the future.40 One or two struts (1–2 mm) should be positioned into the aorta and adequate dilatation should be done without causing dissection of aorta. It is advisable to avoid the use of Amplatzer (Abbott) guiding catheters in ostial lesions. Short-tipped guiding catheters are appropriate in these circumstances. The bumper wire technique – often referred to as the floating or sepal wire method – uses a second guidewire inserted into the aortic root to mark the ostium and prevent the guide catheter from prolapsing past the target ostial lesion.41
Left Main Coronary Artery Bifurcation Percutaneous Coronary Intervention
The approach to distal LMCA PCI is based on vessel anatomy, lesion characteristics and involvement of the branch vessel.
Provisional Stenting Strategy (One-stent Technique)
In this strategy, most commonly LMCA-LAD or main-branch (MB) stenting is done with a wire protecting the LCX or SB. This is followed by POT wherein a short balloon of appropriate size is inflated in the LMCA just proximal to the carina.42 If there is significant compromise of the SB or associated angina or haemodynamic changes, bail-out stenting of the SB may be carried out using T and small protrusion (TAP) or culotte techniques. A step-wise approach, with deployment of second stent, may be used with the SB stent only if suboptimal result is obtained after kissing.43 As outlined in the EBC MAIN study, a repeat POT followed by re-crossing and a repeat kissing balloon inflation is necessary once the second stent has been implanted.29
There are currently no studies that show the benefits of extending a stent into the ostium when the proximal LMCA is healthy. There is no need to extend the stent all the way to the ostium if the ostioproximal portion of the LMCA is healthy, with satisfactory MLA, and has enough space for performing POT. Dedicated short POT balloons are now available on the market (4–6 mm stent length) for such scenarios.
Two-stent Strategy
This strategy may be considered if the LCX is a dominant vessel, if the LCX ostium has significant disease, if the LCX diameter >2.5 mm or the angle between LAD and LCX is narrow. The T- or TAP-stenting, culotte and mini-crush or double-kissing (DK) crush methods are preferred in this circumstance. DK crush is the best option if the LCX is smaller than the LAD and the bifurcation angle is >70%; otherwise, either culotte or DK crush may be taken into consideration. The use of two-stent procedures should be tactically planned and the operator should be knowledgeable of the obstacles and constraints unique to each approach. Imaging-guided procedures should be used to improve clinical results. Final kissing balloon inflation (FKBI) is mandatory in this technique; failure to perform this is regarded as technical failure since it may jeopardise clinical outcome. Additional POT performed after kissing balloon inflation, known as ‘final POT’, is recommended regardless of the provisional stenting technique.44–46
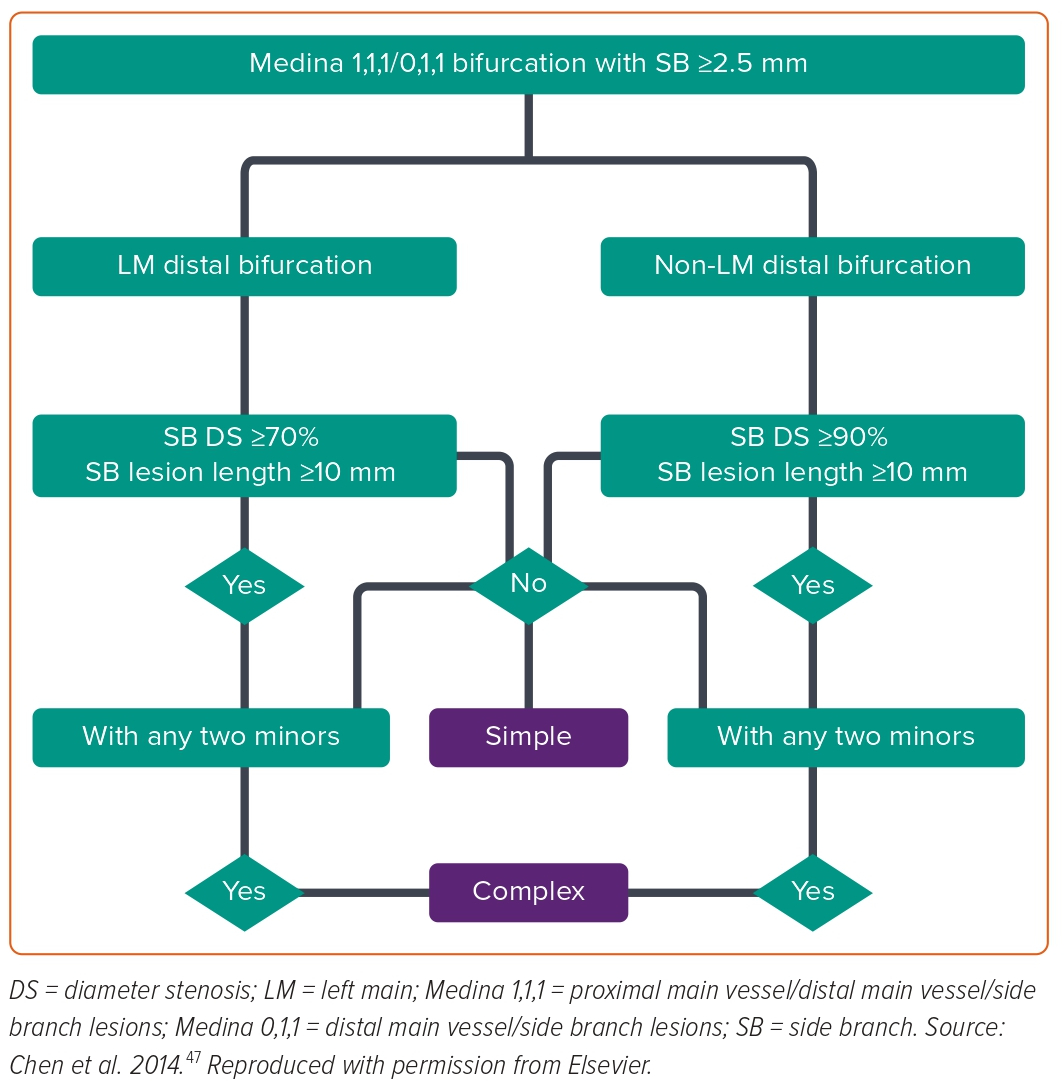
Currently, the most common criteria used to decide on a provisional or two-stent strategy are the DEFINITION criteria. This helps us stratify the lesion based on complexity and plan the appropriate treatment (Figure 3).47,48 Complex LMCA bifurcation lesions are defined as those meeting a major risk factor (SB diameter stenosis ≥70% and SB lesion length ≥10 mm) or any two minor risk factors (moderate to severe calcification, multiple lesions, bifurcation angle <45 degrees, main vessel reference vessel diameter <2.5 mm, thrombus-containing lesions or main vessel (MV) lesion length ≥25 mm).
Two randomised trials have compared the DK crush technique with culotte (DKCRUSH-III) and provisional stenting (DKCRUSH-V) and reported significantly lower target-lesion revascularisation (TLR) and stent thrombosis (ST) with the DK crush technique.49,50 The DK CRUSH-V trial included 482 patients with an ULMCA PCI who were randomly assigned to either the DK crush or provisional stenting technique. The results show that a two-stent DK crush strategy is superior to provisional stenting (with a need for bailout stenting in nearly 50% of patients) for clinical outcomes at 12 months for LMCA PCI, driven by a decrease in TLR and target vessel MI; DK crush had significantly lower rates of TLF (5.0% versus 10.7%; p=0.02) and lower rates of definite/probable ST (0.4% versus 3.3%, p=0.02). At 1 year follow up, for patients with complex LMCA bifurcation disease, the number needed to treat with DK crush to prevent TLF was a remarkably low number of nine. At 3 years follow up, patients undergoing DK crush continued to have significantly lower rates of TLF (8.3%) compared to provisional stenting (16.9%). This suggests that an elective two-stent approach such as DK crush, instead of relying on bail-out stenting, is better in achieving optimal clinical outcomes for complex bifurcation lesions.
The recently published EBC MAIN study randomised 467 patients with true ULMCA bifurcation lesion to a step-wise provisional strategy versus an upfront two-stent strategy.29 Composite endpoint of all-cause death, any MI and TLR was similar in both groups at the end of 12 months was 14.7% (provisional) versus 17.7% (two-stent strategy; HR 0.8; 95% CI [0.5–1.3]; p=0.34). In this study, only 5% of the patients underwent a DK crush procedure. In the two-stent approach arm, a significant number of patients had culotte followed by T/TAP method.
Brief Overview of Various Stenting Techniques
T and Protrusion Technique
The TAP technique is usually considered when the angle between LAD and LCX is >70 degrees.51 It is also used if the crossover technique compromises the ostium of LCX, resulting in suboptimal results. Both LAD and LCX are wired. A stent is positioned in the LCX and the balloon catheter in LMCA-LAD after pre-dilatation of lesions. The LCX stent is deployed with the proximal margin protruding into LMCA fully covering its ostium. After the wire and balloon are removed, the stent is crushed with the pre-positioned balloon in the LAD-LMCA. After the balloon is removed, a stent is deployed in the LM-LAD across the LCX. A wire is re-advanced into the LCX for FKBI.
In provisional TAP, after pre-dilatation of LMCA, an LMCA-LAD stent is deployed. If the ostium of LCX is compromised, a wire is advanced into LCX through distal stent strut followed by balloon dilatation to dilate the stent strut at the origin of the LCX. Then a stent is positioned in the LCX with proximal margin protruding into the LM to completely cover the ostial LCX while a balloon is positioned in the LMCA-LAD. The stent balloon is pulled back and FKBI is performed by inflating the pre-positioned balloon in the LMCA-LAD simultaneously to complete the procedure.
Double-kissing Crush
The DK crush technique is considered in true bifurcation lesions, planned for two-stent strategies where the angle between LAD and LCX is <70 degrees.47 It can be performed using a 6 Fr guide in a step wise fashion, but a 7 Fr is usually preferred for operator ease. After wiring both the vessels, the LMCA- LCX stent is deployed with 1–2 mm protruding into the main lumen. Then the wire is removed and the stent is crushed with a balloon catheter positioned across the LMCA-LAD. The LCX artery is rewired through proximal struts and first kissing is performed. The proximal stent may need post-dilatation using a balloon sized 1:1 to the proximal MV to achieve sufficient proximal stent expansion since the MV stent is sized to match the distal MV diameter. Rewiring of the SB may cross behind the MV stent if the proximal portion of the MV stent is not sufficiently expanded, which might result in MV deformation. The aperture of the struts that cover the SB ostium is also enlarged by sufficient expansion of the proximal MV. The LMCA-LAD stent is then deployed followed by rewiring of the LCX and final kissing balloon inflation/second kissing. To preserve circular geometry across the bifurcation and reduce the SB ostium strut obstruction and stent mal-apposition, POT is repeated at the end of the DK crush technique. To prevent disrupting the bifurcation stent design, it is advisable to retain the distal balloon marker for the re-POT just proximal to the carina in contrast to the initial POT.52
Culotte Technique
The culotte technique can be used when the angle between LAD and LCX is shallow (<70 degrees) and the vessels are usually of similar diameters.53 The decision for MB/SB is at the operator’s discretion. After pre-dilatation, the stent is deployed from the LAD to LMS across the LCX ostium, with the proximal portion positioned in the LMCA followed by POT with a larger balloon at the LMS portion. The LCX is then re-wired and the balloon is inflated at the ostium of the LCX to open the stent struts to facilitate stent advancement into the LCX. The second stent is advanced into the LCX with the proximal portion positioned in LMCA overlapping the first stent and deployed after removing the LAD wire. Then wire is re-advanced through the stent struts into the LAD to perform FKBI and re-shape the carina. The sequence of stenting the LAD can be reversed if LCX is large and there is severe angulation. The wet model images of the final outcomes of these techniques are provided in Figure 4.
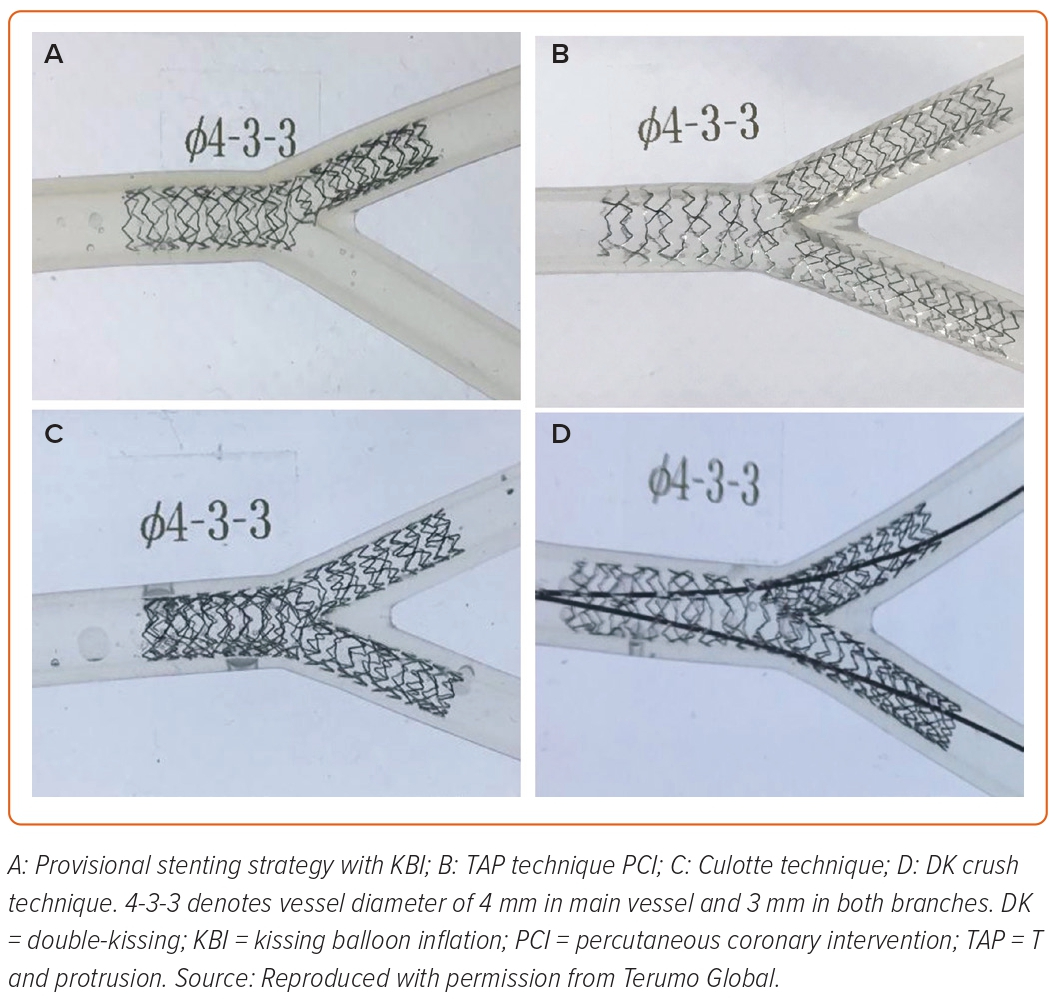
Double-kissing Mini-culotte Technique
The DK mini-culotte technique was developed to overcome the inherent drawbacks of the conventional culotte technique.54 It is characterised by pre-imbedding a balloon in the MB as necessary, then stenting the SB or the smaller branch first, mini-protruding the SB stent into the MB and DK balloon inflation, i.e., an initial and a final kissing balloon inflation. This technique is beneficial for the management of true coronary bifurcation lesions.
Left Main Percutaneous Coronary Intervention in Acute Coronary Syndrome
ACS with a lesion in the LM (LMCA-ACS) occurs infrequently, but often leads to severe haemodynamic compromise and sudden cardiac death despite significant improvements in care processes.55–57 ACS related to ULMCA disease is particularly challenging and represents a distinctive subset. In this setting, PCI might be the only therapeutic alternative, particularly in presence of haemodynamic instability, such as cardiac arrest or cardiogenic shock (CS).
The survival of patients with ULMCA disease presenting with ACS depends on different variables and is lowest in those with CS. It is important here to emphasise the utility of mechanical circulatory support devices (intra-aortic balloon pump, Impella device, extracorporeal membrane oxygenation, etc.) prior to PCI in this group of individuals. Otherwise, it is possible that the patient suffering from haemodynamic compromise will deteriorate or arrest during the procedure itself. These devices help in left ventricular unloading to enable coronary revascularisation.58,59
The SALvage study included 134 patients presenting with ACS and LMCA disease, classified into two arms, ST-elevation MI (STEMI)/CS and non-STEMI (NSTEMI)/unstable angina (UA), and followed them up for 6 months after LMCA PCI.60 It was noted that 64% of lesions were distal and 25% underwent two-stent technique. The primary endpoint of all-cause mortality was 44% versus 6% in the non-CS group (p<0.001). This study suggested ACS due to critical ULMCA stenosis is associated with higher mortality, even after successful PCI. In this study, more bare-metal stents than DES were used in the STEMI group. Overall, 16 patients had complete LMCA occlusion.
Palmerini et al. published the clinical outcome of patients treated by ULMCA-PCI for ACS (n=611) in comparison to stable angina (n=490). ACS was associated with a two- to three-fold increased risk of cardiac mortality and MI during a 2-year follow-up. Patients with STEMI and those in CS were excluded from the study.61
A retrospective registry from six centres in Japan involving consecutive patients undergoing LMCA PCI included 1,500 patients with ULMCA stenting for LM ACS (ACS with shock: 115 patients; ACS without shock: 281 patients) and stable coronary artery disease (1,104 patients). The cumulative 180-day incidence of death was markedly higher in the ACS with shock group than in the other groups (49.5%, 8.6% and 3.3%, respectively; p<0.0001), but mortality beyond 180 days was not significantly different among the three groups (30.2%, 20.4%, and 19.5%, respectively; p=0.65).62
The 2011 American College of Cardiology Foundation/American Heart Association/Society for Cardiovascular Angiography and Interventions guidelines recommend PCI for LMCA disease for those patients presenting with STEMI requiring prompt revascularisation (class 2a recommendation) and UA/NSTEMI who are not suitable for CABG.63
Further to this, there has been no specific mention about ACS-LMCA scenario in the updated guidelines. In the above-mentioned studies, ULMCA PCI was considered as a valuable therapeutic option at that moment. With the current use of DES and imaging guidance, we expect the outcomes may slightly improve; a randomised study of ULMCA ACS patients undergoing PCI in the current era is warranted.
Conclusion
In recent years, a growing number of operators have started to perform LM PCI in patients with LM disease. Correct procedures, an adjunct strategy and intravascular imaging have all improved patients’ clinical outcomes. We can achieve superior outcomes by understanding the anatomy, intricacy and importance of the LMCA and by maximising the application of procedures that have been shown to be more effective than others. However, a subset of individuals with LM disease would still require surgery.
Recent revascularisation recommendations advocate the use of a heart team and patients weighing-up their alternatives before making an educated decision. For the benefit of the patient, institutions should adapt a heart-team approach for patient selection based on anatomical and clinical characteristics. 











Comments