Coronary artery fistula (CAF) is a false vascular channel; that is, a direct communication of the epicardial coronary artery with one of the four chambers or the major vessels of the heart or chest. It is one of the rare coronary anomalies and can be congenital or acquired, and comprises 0.8% of all coronary anomalies.1 Its incidence is only 0.002% in the general population and 0.1% in all cardiac catheterisation patients.2 It is present in approximately 1% on autopsy examinations and in 4–15% of cases of young sudden cardiac death.3 It represents half of all coronary congenital anomalies with no gender, race or age predisposition.1–3 CAF can be large (>250 mm), ectatic and dilated on presentation or can become enlarged over time. Since its first description by Krause in 1865, and later the classical description of the triad for CAF (murmur, communication of arterial and venous channel, and tortuosity of vessel) by Haller and Little, its importance in cardiac physiology has increased.4,5 With advances in imaging modalities and cardiac diagnostic tools such as CT coronary angiography (CTCA), a higher frequency of cardiac anomalies including fistulas are being detected than previously seen using invasive angiography (IA).
The long-term cardiovascular sequelae of CAF are still to be explored. Failure to recognise them may lead to incorrect diagnosis and altered treatment, both in the cardiac catheterisation laboratory and in surgical suites. The greatest challenge is that they can occasionally cause severe critical symptoms or events that warrant treatment. Most coronary fistulas are asymptomatic; however, symptoms can lead to heart failure, endocarditis, conduction abnormalities and myocardial steal, leading to angina and dyspnoea.6
Still, to date, limited literature exists on the long-term implications for the coronary haemodynamics, pathophysiology and management in asymptomatic and/or symptomatic patients. In the last decade, with the increasing global surge in coronary interventional procedures, coronary artery bypass grafting and chest radiation, the rate of identification of acquired or sporadic causes of CAF has risen and is expected to rise further in the future. In this literature review, we focus on the origin of fistulas and the existence, classification, pathophysiology, importance, diagnosis and treatment of CAF.
Origin and Causes of Coronary Artery Fistula
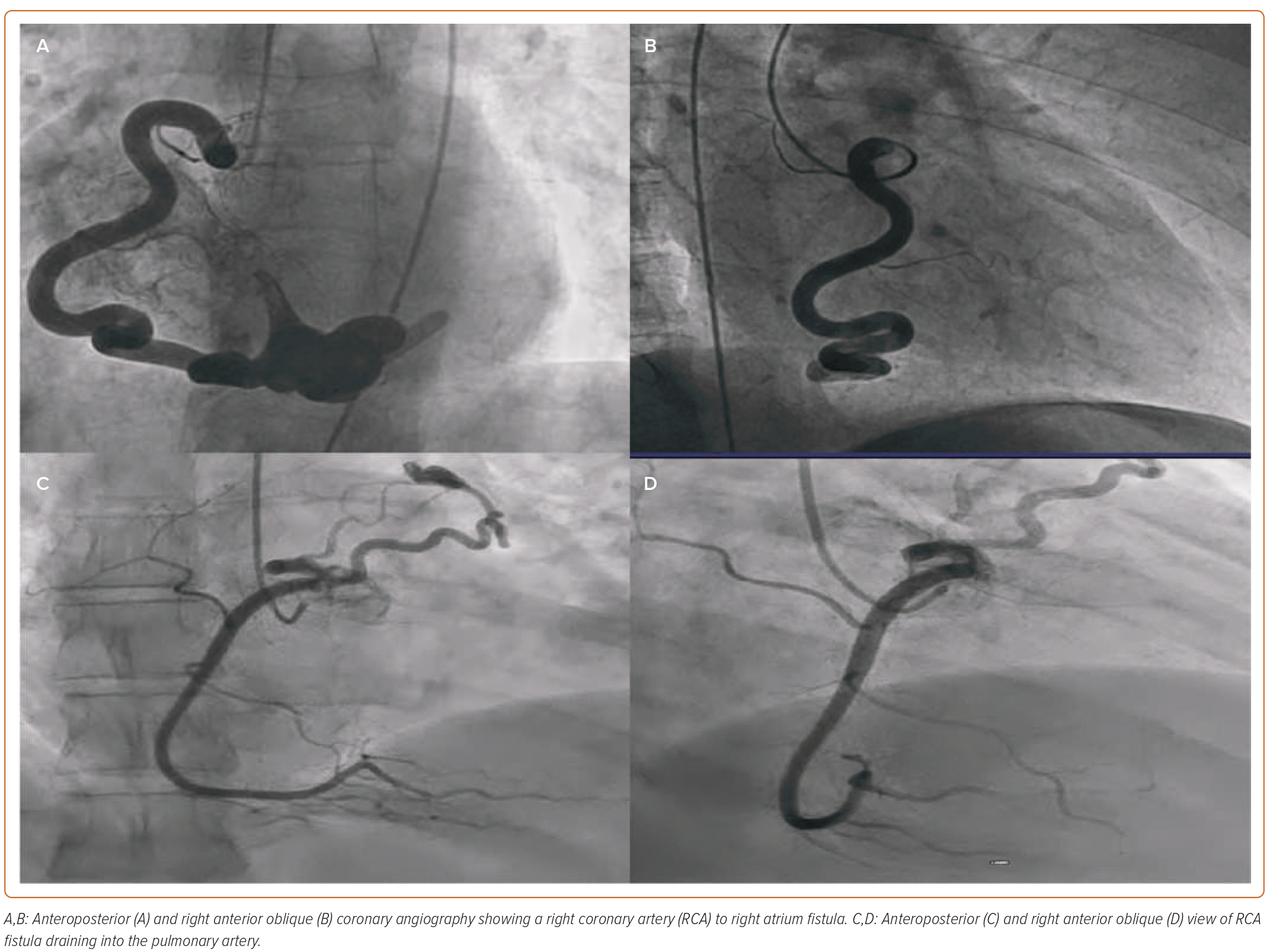
CAF can be congenital or acquired, with 90% of the cases being congenital (Supplementary Table 1).2 Normal coronary arteries originate in the aorta and terminate in the myocardial bed. The communication between the arteries and chambers of the heart or great vessels takes place through the sinusoids, which transform later in life into the capillary network.7 During embryonal life, the primitive myocardium during early fetal development is supplied by sinusoids and is connected to the primitive tubular heart. These sinusoids are later transformed into the basilar vessels and capillaries. Failure of the regression of the sinusoids leads to fistulous communication between the coronary arteries and the cardiac chambers (Figures 1A and 1B). As well, there can be a remnant of primordial connections between the coronary arteries and mediastinal vessels (or bronchia or mediastinal or superior vena cava connections), which can cause CAF (Figures 1C and 1D).8
Acquired or sporadic (Supplementary Table 1) causes of fistula, although usually rare (10%), are becoming more frequent as increasing numbers of patients undergo percutaneous coronary intervention, coronary artery bypass graft surgery, and radiation procedures to the chest for medical reasons. Diseases such as MI and vasculitis can lead to CAF formation during the healing phase. Due to the increasing number of these procedures, the frequency of identification of sporadic causes is expected to increase yearly.
Pathophysiology
The main pathophysiology of CAF is the drainage of high-pressure blood in the arteries into a low-resistance venous system through a channel that bypasses the natural low-pressure small arteriolar and capillary network in the myocardium.8 Its resistance is dependent upon the fistulous connection and the site of fistulous termination. Hence, accurate assessment of the origin and drainage site is necessary to identify steal, which results from decreased perfusion of the myocardium distal to the fistula, secondary to a coronary steal phenomenon.9 The steal phenomenon is responsible for angina, which usually occurs in conditions of extreme oxygen demand such as exercise. Gradients across the artery and the drainage area determine the extent of shunt in the coronary fistula.
The steal phenomenon in CAF is complicated. Steal can occur as ‘persistent steal’ or as ‘episodic steal’. Persistent steal is when large amounts of blood are diverted into a large fistula tract, resulting in deprivation of nutrient-rich blood feeding the coronary artery or collateral circulation, resulting in myocardial ischaemia. Episodic steal is physiological and occurs during times of high oxygen demand (such as exercise) when the nutrient artery is deficient during stress and more blood is diverted into the fistula tract. Steal is very difficult to diagnose because routine stress imaging is unable to access the degree of ischaemia in these cases, given that vasodilators have little or no effect on the fistulous artery. Any right-to-left shunt would lead to increased pulmonary hypertension and volume overload with ventricular failure, leading to high output cardiac failure. In contrast, a left-to-left shunt would increase volume in the left ventricle only but could also lead to high output failure. Fistula can be large on presentation or tend to enlarge over time.10
Sometimes CAF exists together with underlying atherosclerotic coronary artery disease and the pathophysiology depends upon the exact origin of CAF, that is, whether it occurred before or after coronary atherosclerotic stenosis.11 The pathophysiology of myocardial ischaemia differs significantly in the two situations. In the former situation, mostly a left-to-right shunt is responsible for ischaemia secondary to the steal phenomenon, while in the latter situation the distal myocardium is deprived of oxygen usually secondary to right-to-left shunt by oxygen-deprived pulmonary blood on top of the stenosis, leading to further decrease in oxygen delivery to the distal bed of the myocardium.12
Clinical Importance and Consequences
After establishing the definition, incidence, and pathophysiology of CAF, the next most important question is the evaluation of its clinical significance. The fundamental question in this regard is whether CAF is the cause of the clinical symptoms and whether it can result in unfavourable clinical conditions. Mostly CAFs are asymptomatic and the artery providing the vital blood flow to the myocardium is rarely impaired, but they can cause haemodynamic compromise and complications depending upon their size, site of origin and destination.13 Patients can present with vastly different symptomatology. Symptoms of CAF range from mild dyspnoea, fatigue, chest discomfort, dizziness, syncope, and angina to severe heart failure, pulmonary congestion and MI, all depending upon the severity of the steal and the shunt pathway.13–16 Recent cumulative evidence suggests that fistulas remain asymptomatic in first two decades of life but with advancing age the majority of fistulas become symptomatic, especially in the fifth or sixth decades of life.13 For age <20 years, only one-fifth of the fistulas are symptomatic, while more than two-thirds of the fistulas are symptomatic after the age of 60 years. In rare cases, in patients with larger fistulas a cardiac murmur is audible on cardiac auscultation. Murmur usually occurs as a crescendo decrescendo pattern throughout systole and diastole, like any other fistulous murmurs in the body. Most fistulas are found incidentally during conventional IA or CTCA performed for various cardiac or similar reasons. Smaller fistulas are mainly asymptomatic and are managed conservatively while larger fistulas are usually referred for further evaluation based on unexplained anginal complaints or loud continuous murmur. Valvular regurgitation, infective endocarditis and muscle dysfunction, especially of the papillary muscles, have been reported.sup>14 Haemopericardium and cardiac rupture secondary to CAF, although rare, have also been reported.15 Antibiotic prophylaxis is recommended once the fistula is diagnosed.2,11,16
Diagnosis of Coronary Artery Fistula
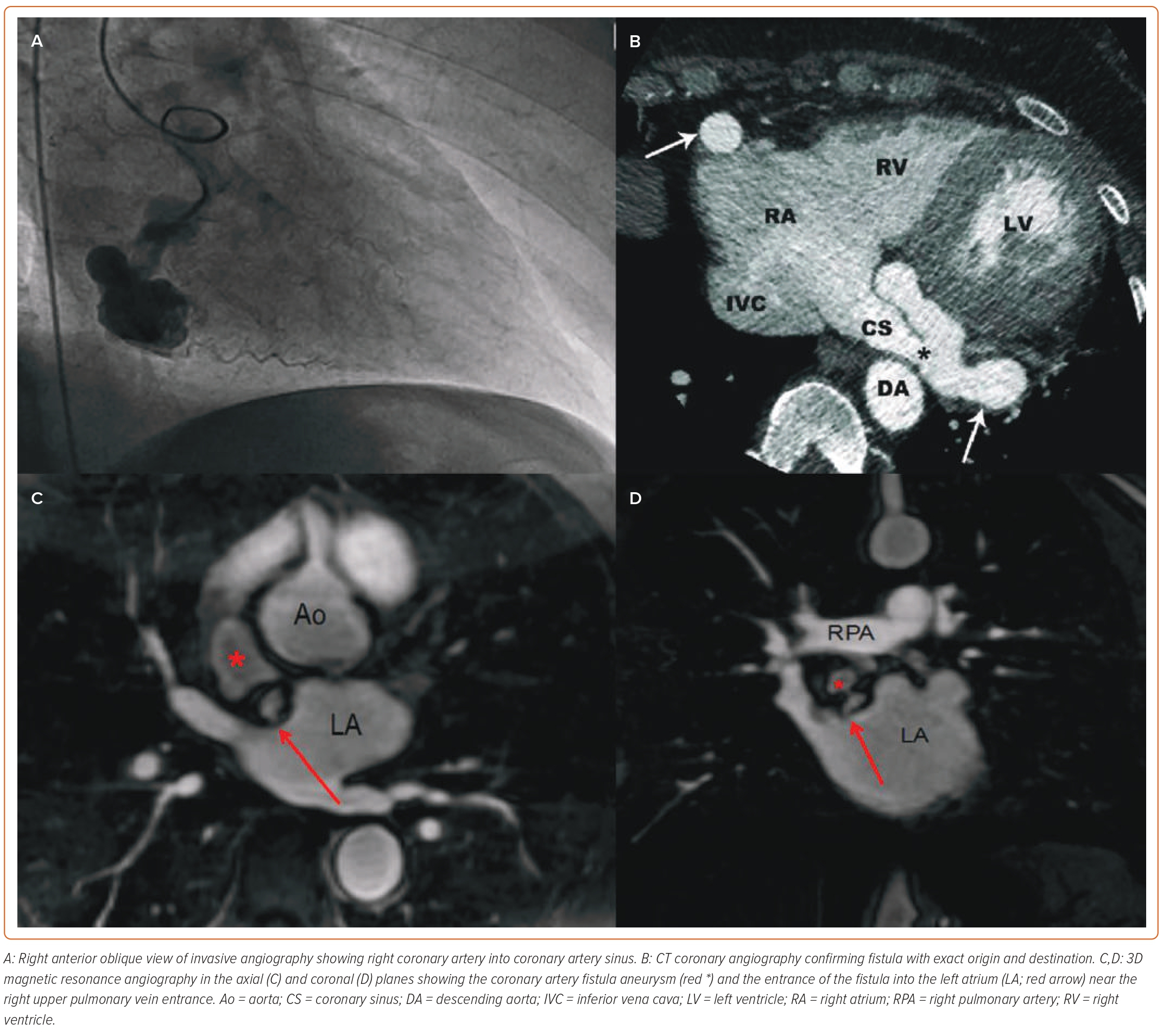
Various imaging modalities can be used for the diagnosis and assessment of CAF including conventional IA, CTCA (Figures 2A and 2B) and MRI (Figures 2C and 2D).17–19 ECG and chest X-ray are normal in the majority of cases or are of little benefit. There are no conventional ECG changes in CAF, although various ECG abnormalities are observed.20 ECG may show signs of left or right ventricular hypertrophy depending upon the shunt. Right heart strain and different conduction abnormalities have been seen in cases of fistula. Pulmonary congestion is seen in some cases of fistula on chest X-ray, especially in cases of severe left-to-right shunt.20
The role of routine transthoracic echocardiogram (TTE) is important but colour Doppler and trans-oesophageal echocardiogram not only help in pressure estimation in cases of shunt but also in delineating the flow in the aneurysmal coronary artery or the recipient chambers.21 However, the exact origin and anatomy are often difficult to delineate with echocardiogram, but microbubble contrast material often helps (Supplementary Figure 1).22 Echocardiography is safe for pregnant women and children because it avoids ionising radiation and intravenous contrast. Echocardiography can also help in detecting any other associated acquired or congenital cardiac defects, masses and/or thrombotic lesions associated with CAF. It also helps in shunt calculations. Given the substantial improvement in TTE resolution, new studies suggest that echocardiography can be of great importance in diagnosing the valve associated with CAF. Despite this, the diagnostic accuracy is still 35–50%.21,22
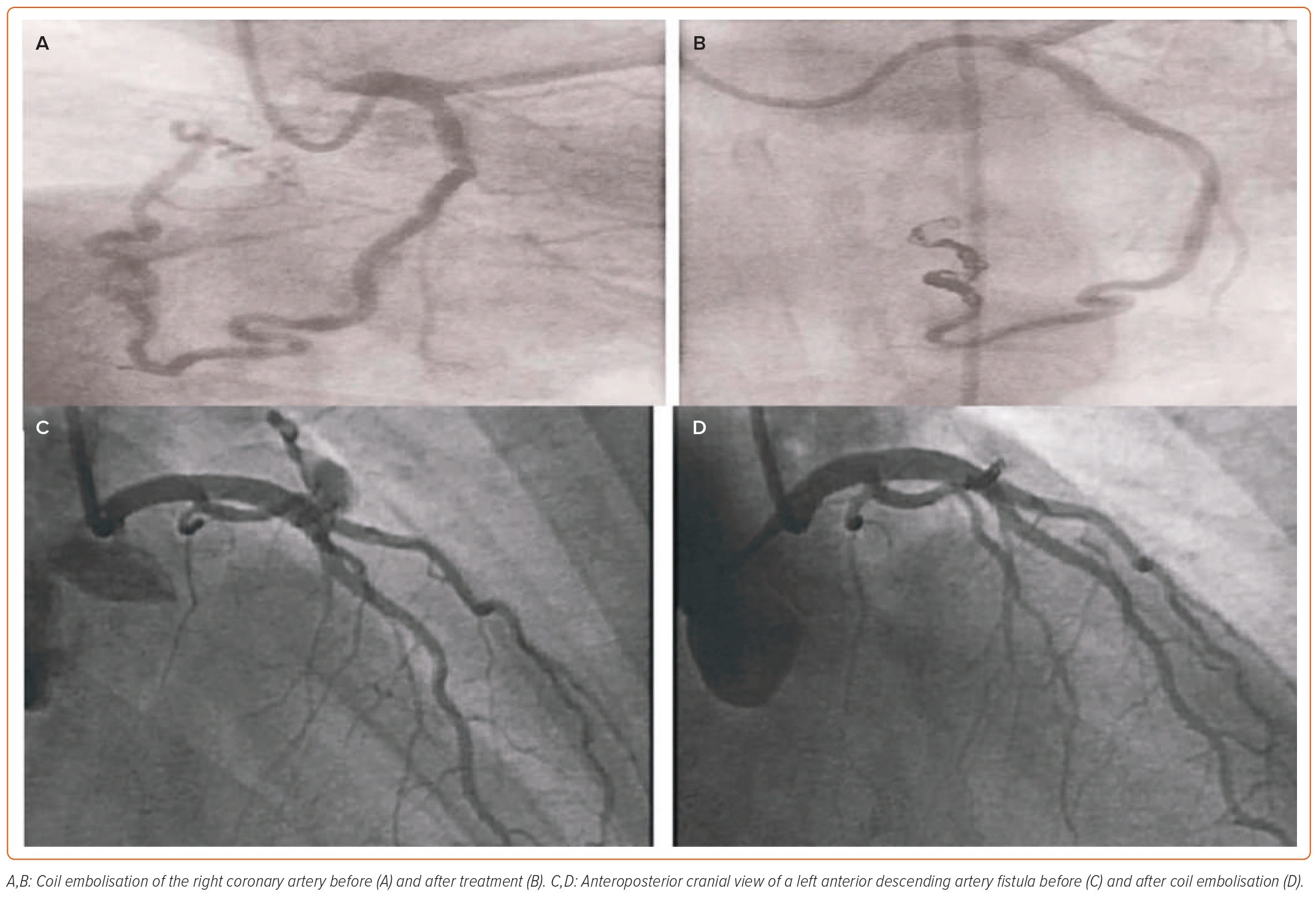
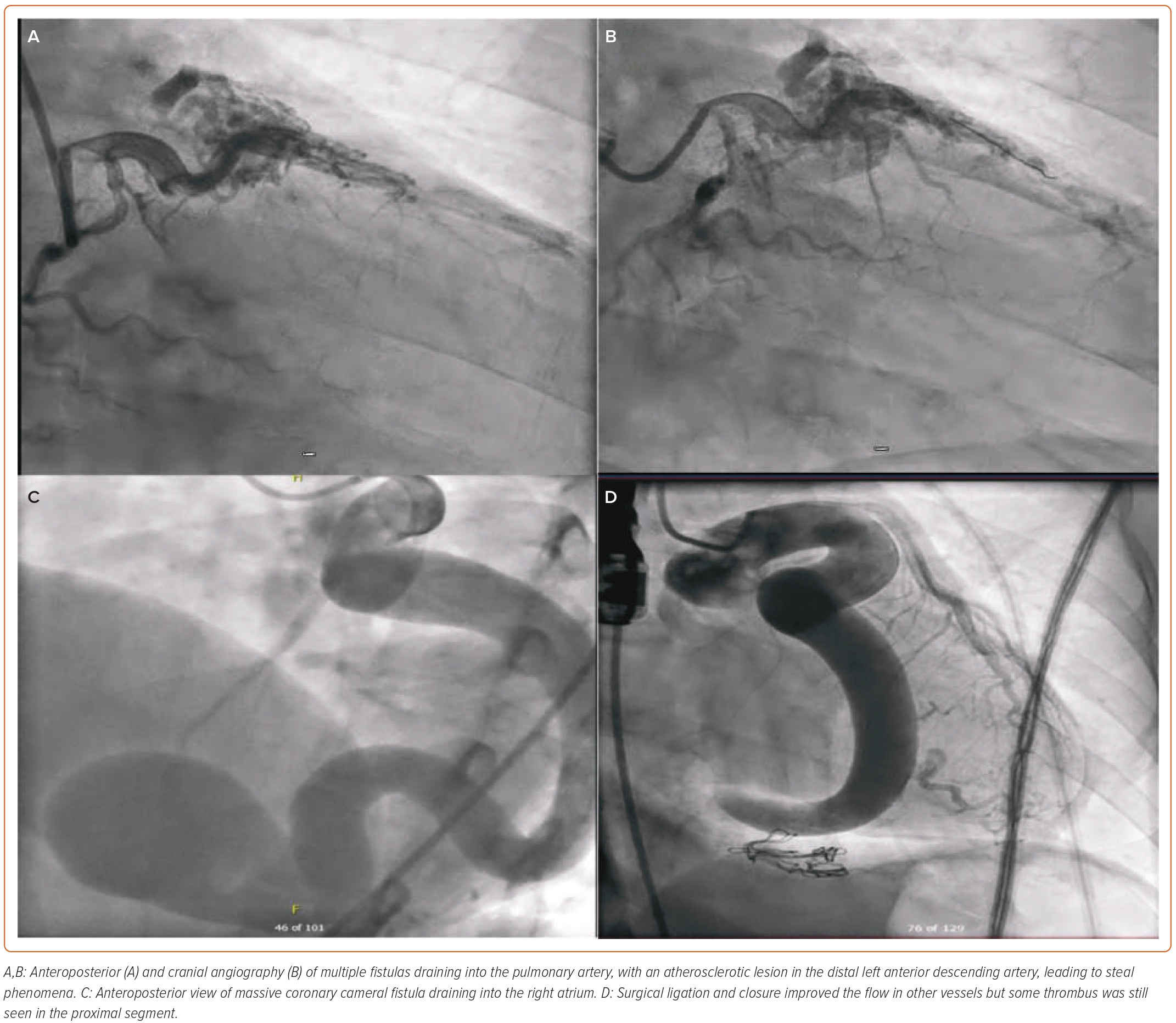
IA, traditionally the gold standard investigation for CAF, is helpful in precise visualisation of the anatomy and in locating the origin and visualising the insertion of fine vessels and other structural defects associated with fistulas.23,24 It provides important information regarding haemodynamics with high temporal and spatial resolution.24 In addition to the diagnosis, it helps in decision-making by enabling consideration of therapeutic embolisation in the choice of treatment for CAF (Figure 3).25 However, as a reference standard, IA is invasive and it involves the risk of complications. Furthermore, it is a 2D still image of a 3D structure, which might not yield sufficient information in some cases.
Given recent advancements in imaging, MRI may serve as alternative to CTCA and conventional IA, particularly in individuals who cannot undergo repeat imaging studies (Figures 2C and 2D).26 It does not involve contrast and ionising radiation. However, MR angiography takes longer to perform than CTCA and has a relatively lower spatial resolution.27 Extracardiac structures and distal coronary arterial courses are not clearly visible on MR angiography, which is the major drawback with this modality. Moreover, not only is it contraindicated in patients with metal implants such as pacemakers and defibrillators, it also cannot be performed in patients with claustrophobia.26,27
CTCA has become the gold standard diagnostic evaluation tool for CAF because of the higher temporal and spatial resolution compared with other imaging modalities.18,19 It has a shorter acquisition time and also has higher temporal and spatial resolution.10,19 With 3D reconstruction and multiplanar slices, the CAF’s origin, course and destination can be examined. Its potential role in treatment, especially in complex, symptomatic and long CAF, is much higher than that for IA (Supplementary Table 2). One of the disadvantages of CTCA is that it involves radiation exposure (which could be reduced by reconstruction protocol along with ECG gating).28
Myocardial perfusion imaging with nuclear testing is also useful in cases of symptomatic CAF because it not only delineates the anatomy but also helps in perfusion testing and myocardial ischaemia.29 It can also be used in complex cases for grading the severity of ischaemia and for confirmation of morphological ischaemia by CAF that has been identified on other imaging modalities such as CTCA.
The role of intravascular ultrasound is also defined and is particularly helpful in certain cases in assessing the flow dynamics and treatment in percutaneous closure.30 It is helpful in delineating clots in fistulous tracts, thrombus, aneurysmal segments and measuring vessel size for therapeutic reasons. The role of optical coherence tomography (OCT) has not been established in the literature but can be of prime importance in selected cases in which clear visualisation of the outer wall or intima is required. Pressure wire measurement can also be used to assess the pressure gradient across the fistulous tract but it is seldom used, and the available evidence is limited with regards to its use in CAF cases.
An underutilised diagnostic utility is right heart catheterisation (RHC). RHC should be performed in left-to-right-sided cases of CAF for the assessment of shunt calculation, degree of volume overload and to establish a baseline evaluation before consideration of treatment options. Very little literature is currently available on the utility of RHC in CAF. It is helpful in therapeutic decision-making and symptom management. We strongly recommend that RHC becomes an integral part of the diagnostic evaluation of CAF, especially in cases of large shunt with volume congestion, and to establish the baseline before therapeutic options.
Despite the advancement and wide range of available imaging modalities, still multimodality imaging is required in majority of cases and case base techniques are demonstrated in literature to get the desired result with reduce amount of radiation dose especially in radiology.
CT Angiography Protocol
The goal of CTCA is to provide maximum information on the CAF via still images with the lowest possible radiation dose.31 All images should be obtained with the patient’s heart rate at <75 BPM and reviewed with ECG gating.32 This should be achieved with β-blockers unless they are contraindicated. Radiation dose should be adjusted for heart rate and BMI to decrease the amount of radiation, and reconstruction methods should be enabled while preserving the image quality.28 All of these measures are necessary and helpful in obtaining the relevant information with the least possible side-effects.
Most of the CT protocols are institution specific but we recommend a scan covering the chest wall superiorly up to the aortic arch and up to the level of the heart with a wide field of view. The CT scanner should be a 256 section scanner with 128 × 0.625 mm detector collimation.<>33 The tube rotation time should be 272 ms. Iodinated contrast material should be injected according to body weight (1 ml/kg + 5 ml) at 5 ml/s followed by 60 ml of saline flush.34 Hydration should be considered before and after the procedure in the case of chronic kidney disease patients with low renal clearance. Tube kilovoltage should be 100 kVp in patients with BMI <25 kg/m2 and 120 kVp for BMI >25 kg/m2.33,34 Reconstruction of the imaging with multiplanar 3D volume rendering with curved maximum intensity projection imaging along the fistulous tract should be done. Prospective ECG gating should be performed. The majority of the drainage sites are above the level of carina, and a scan including the chest wall is meant to cover the origin and destinations of most fistulas, which are mainly in the pulmonary, coronary artery, systemic vein and superior vena cava. When contrast is seen in the drainage site on CT angiogram it is called a CT shunt.35
Classification of Coronary Artery Fistulas
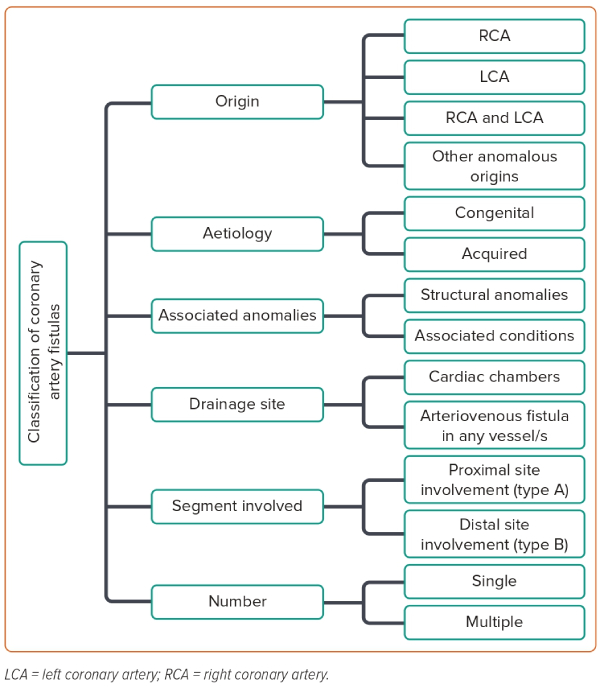
There is no single universal classification system for CAF; instead there are various classification methods, based on the complexity, drainage site, origin, number of tracts, recent coronary anatomy and the draining artery or the accompanying congenital abnormalities.36,37 Figure 4 shows a classification algorithm for CAF.
Any coronary artery can be an origin of CAF but the right coronary artery (RCA) accounts for 50–55% of the cases while the combined left coronary artery (LCA) and RCA account for 5–20% of the cases.36,37 Based on the draining site, CAF is classified into two types: coronary cameral fistulas and coronary arteriovenous fistulas.2 Coronary cameral fistulas drain into one of the cardiac chambers while the coronary arteriovenous fistulas drain into any segment of the pulmonary or systemic circulation. Most of the fistulas drain to the lower pressure right side of the heart rather than to the high-pressure left side of the heart.38
Of interest, CAFs are classified into single coronary fistulas, which account for 90% of the cases, and multiple coronary fistulas, which account for 10–16% of the cases.39 Morphologically CAFs are classified into simple and complex.22 Simple CAF has a single origin and drains through a single tract while complex CAF has multiple fistulous connections (Figure 5A, B). Based on congenital abnormalities, 5–30% of fistulas are associated with other congenital anomalies while 55–80% of cases are isolated CAFs.40 The common congenital anomalies associated with fistulas are ventricular septal defect, tetralogy of Fallot, patent ductus arteriosus, atrial septal defect and pulmonary atresia with an intact ventricular septum.41
Differential Diagnosis of Coronary Fistula
Any dilatation of the coronary artery could be a differential diagnosis for CAF including an anomalous origin, vasculitis, coronary artery aneurysm and ectasia secondary to atherosclerosis. Dilation and ectasia are usually reported in children with a postviral syndrome or flair up of a vasculitic process. Kawasaki and Takayasu arteritis are the main differentials. Kawasaki disease is a vasculitis that affects medium-size vessels, especially in children. In young patients there is a classic angiographic bead-like appearance, especially with multifocal ectasias and stenosis.42 Takayasu arteritis is a medium-size vessel arteritis that causes aneurysmal segments in the aorta and large vessels, and it can cause dilatation or stenosis very similar in appearance to fistula.43 It can be differentiated from CAF in the absence of a fistulous connection to the coronary artery. Bland-White-Garland syndrome is a rare cardiac congenital anomaly and one of the differential diagnoses of CAF. It is associated with anomalous origin of the LCA from the main pulmonary artery (also known as ALCAPA syndrome).44 The presence of collaterals between the dilated RCA and LCA distinguishes the infant type ALCAPA from the adult type. In the infant type there is no collateral flow from RCA to LCA and the myocardium is deprived of oxygenated blood.
Management of Coronary Artery Fistula
Management of the CAF depends upon its origin, destination, size, presence of symptoms, age and associated cardiovascular abnormalities.2 Asymptomatic patients are monitored in the majority of cases and do not require any treatment. Very rarely, in 1–2% of the cases, spontaneous closure occurs, and these patients are monitored on a regular basis. Acute coronary syndrome patients are managed with antiplatelets, antibiotic prophylaxis and monitored for complications.9 Large and moderate size CAFs with symptoms such as arrhythmia, ventricular dysfunction or myocardial ischaemia are a class 1 recommendation for closure according to the European Society of Cardiology and American Heart Association guidelines.225
For patient and procedure safety, the first and most important step in the management of fistula is the identification of the origin and destination of CAF, to ensure the safety of the adjacent vessels during the index procedure.2,45 Clinically the drainage site is more important than the origin, given that low-pressure shunts lead to dilation, aneurysm and symptoms. The more distal the shunt is, the more severe is the steal phenomenon and the more symptomatic the patient. Treatment decisions are based on the complexity, number of fistulas and the tracts, which will determine whether surgery or a non-invasive approach is appropriate.18,25,45
Proximal CAF can be closed near the origin or destination depending upon the involved myocardial bed while distal CAFs are closed near their drainage site, and the occluder device is placed as distal as possible to save the proximal myocardial bed.25 In cases of extreme tortuosity or smaller calibre, surgical ligation is preferred because of deliverability issues.
Transcatheter Approach
Percutaneous transcatheter treatment is a minimally invasive alternative treatment for closing CAF compared with surgical ligation.46 It is indicated in symptomatic patients who have a narrow single CAF, mostly proximal in origin, with no other associated cardiac or congenital anomalies (Figure 3). It is also preferred in very young and elderly patients to avoid perioperative complications. Various percutaneous techniques can be used for CAF closure, such as vascular plugs, covered stents, umbrella device, detachable balloons, coils and ductal occluders.2,25 Establishing the chest anatomy before percutaneous treatment is highly advisable. We recommend RHC studies and pressure assessment in all shunt cases before and after the procedure. Shunt calculation is necessary for procedural success and for symptom assessment at subsequent follow-up.47
Percutaneous management is largely dependent on the site of the CAF (i.e. whether it is proximal or distal). In proximal CAF cases, percutaneous closure is generally performed at the proximal end, near the origin or extremely distal to the drainage site. For distal fistulas an occluder device is placed as distally as possible so that the flow of the proximal artery is not compromised, or that of the branches supplying the myocardium.48
Failure of the percutaneous procedure is observed in cases of larger CAF. Distal coil migration, incomplete fistula closure and complete closure of native vessel blood supply with myocardial ischaemia are remote possibilities.48 Some of the complications include dilatation, aneurysmal segments, migration of the clips, thrombus formation and aneurysm of other native coronary arteries.49 Some larger fistulas do contain thrombus (Figures 5C and 5D), especially in elderly patients if they are not taking anticoagulation.49 Thrombus can increase and lead to acute MI. Some patients can develop secondary conduction abnormalities during transcatheter closure.50 If the coil is placed distally, full closure of the fistula or of the small branches might not occur and could lead to incomplete occlusion. With the transcatheter approach, especially in the case of coils, there is a small risk of infective endocarditis.14 Percutaneous coronary intervention for atherosclerotic coronary artery is extremely challenging in the presence of fistula (Figures 5A and 5B). Various intervention techniques and tools can be used in these complex cases for better visualisation during this procedure.
Closure is assessed on subsequent angiography or CT, which usually shows the shunt contraction sign. The sign might not be present, although there would be some degree of shunt visible, therefore it is possible that shunt may be present although it is not visible on invasive CT, possibly because of contrast enhancement timing or artefacts due to other issues.49 Based on the complexities and nature of the fistula, radiological information with 3D reconstruction and volume rendering is necessary before deciding on the treatment plan for surgery versus the percutaneous option.19 Multiplanar 3D volume reconstruction with careful assessment is strongly recommended.
Surgical Treatment
Surgical treatment is offered to those patients in whom the blood flow in the distal myocardium is compromised, and it is preserved while closing the fistulous tract. Surgical closure is preferred in cases in which surgery is indicated with other associated cardiac anomalies or in those involving multiple fistulas that are tortuous, smaller calibre, aneurysmal and ectatic.2,11,25 Simple ligation of the CAF is the preferred surgical technique.51,52 Other techniques include ligation of both the primary and distal segment with implantation of a bypass graft at the distal target site or closure of the recipient cavity. With evolving imaging modalities and growing experience, the reported morbidity and mortality associated with surgical closure has decreased, however, it is associated with a higher complication rate than the transcatheter approach.53
Follow-up
At present there are very limited guidelines for CAF. We recommend CTCA as a good follow-up, non-invasive tool for assessing CAF (Supplementary Table 2). Recent literature suggests that 10% of the transcatheter surgical CAFs do show some leakage of recanalisation or re-emergence of the fistula.53 Follow-up is also important during the first year for evaluating any residual shunt during the early postoperative period.54 Other reasons for follow-up include procedural complications or treatment failure, which may occur and require some degree of follow-up with imaging.55 We recommend that all treated patients should undergo 6 month–yearly follow-up CT with the protocol as specified earlier.
Conclusion
Although rare, CAF is usually asymptomatic, but serious complications are a possibility depending upon the degree and size of shunt. CTCA with 3D reconstruction and ECG is extremely important to assess the CAF. The delineation of the fistula tract is of extreme importance including site, number, origin, drainage size, shunts, associated cardiac congenital abnormalities and pressure assessments with RHC. We recommend, in selected symptomatic cases, that the cardiothoracic team, radiologist and interventional cardiologist should all be involved in the imaging, especially CTCA, IA and right-sided studies, to decide on appropriate clinical and therapeutic options in the evaluation of CAF. We recommend that radiologists should play an integral part in therapeutic and clinical decision-making in the evaluation of CAFs and their follow-up (Supplementary Figure 2).