Transcatheter aortic valve implantation (TAVI) has become a highly effective and beneficial treatment option for patients with severe symptomatic aortic valve stenosis. The randomized Placement of AoRtic TraNscathetER Valves (PARTNER) trials have shown that (1) TAVI using the balloon-expandable Edwards Sapien valve implanted via the transfemoral (TF) or transapical (TA) route is superior with respect to outcomes compared with standard therapy in inoperable patients and that (2) TAVI is at least non-inferior to surgical aortic valve replacement (AVR) in high-risk patients.1,2 More recently, the randomized US pivotal CoreValve high-risk study has reported that TAVI exclusively via the transvascular (83 % TF, 17 % trans-subclavian) approach using the selfexpandable Medtronic/CoreValve prosthesis is in fact superior to open surgery in such high-risk patients.3
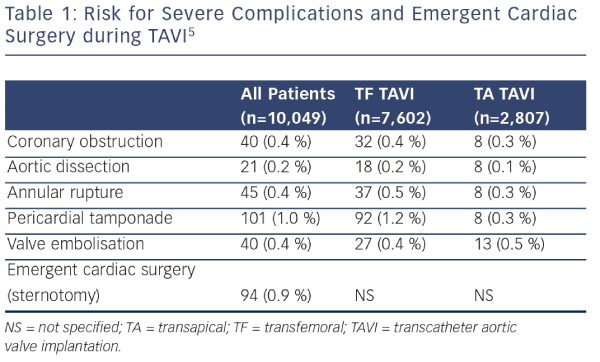
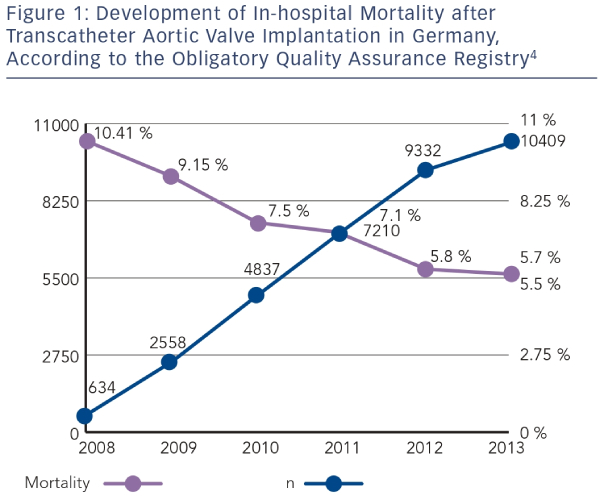
With rapidly increasing experience, TAVI globally has become a safe clinical routine procedure. In Germany, 30-day mortality rates have almost been halved since 2008 (see Figure 1), according to data from the German obligatory quality assurance registry (AQUA registry).4 Although the risk for severe complications such as annular rupture, ventricular perforation, or aortic injury during TAVI is low, ranging between 0.2 % and 1.0 % (see Table 1),5 some of these complications may ultimately require emergency cardiac surgery (ECS).
Risk for Emergent Cardiac Surgery during TAVI
The most recent Valve Academic Research Consortium (VARC)-2 consensus document lists ECS among other TAVI-related complications, defined as any conversion to open sternotomy during the TAVI procedure secondary to any procedure-related complication. In the published literature, ECS is often defined as any cardiothoracic surgical intervention requiring cardiopulmonary bypass and sternotomy for urgent aortic valve replacement, repair of myocardial perforation or aortic injury, or pericardial drainage performed during or within 24 hours after TAVI.6 Nevertheless, definitions may be heterogenous throughout the literature.7 It may further be speculated that in some very-high-risk TAVI cases, ECS is not even attempted due to the anticipated bad outcome. Therefore, comparison of ECS rates between different studies may be hampered.
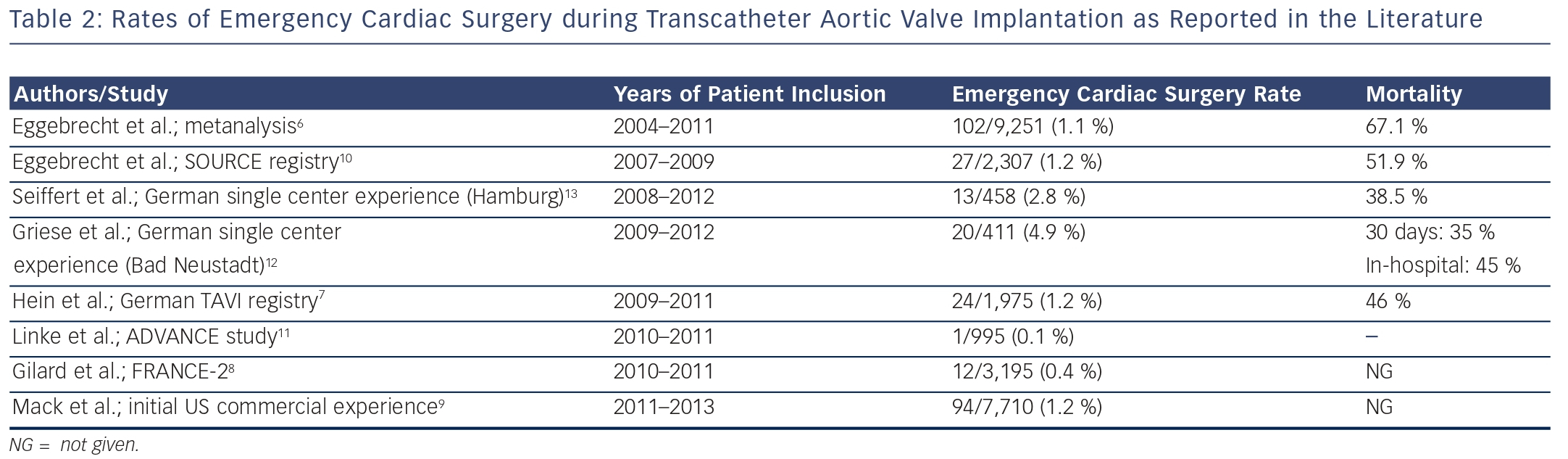
In a meta-analysis of 46 published studies comprising a total of 9,251 TAVI patients, ECS was required in 102 (1.1 %) patients.6 ECS rates were higher among patients undergoing TA TAVI compared with those undergoing TF TAVI.6,7 Contemporary national and international TAVI registries have yielded similar rates of ECS during TAVI. In the most recent report from the AQUA registry comprising 10,409 patients undergoing TAVI in Germany in 2013, ECS with sternotomy was required in 94 (0.9 %) patients.5 The FRANCE 2 registry reported that 12 out of 3,195 TAVI patients (0.4 %) were converted to open surgery.8 In a report from the initial US commercial experience, 94 (1.2 %) out of 7,710 patients underwent conversion to open heart surgery for TAVI complications.9 The international SOURCE registry using the balloonexpandable Edwards Sapien valve similarly reported an ECS rate of 1.2 %.10 More recently, the risk for ECS appeared even lower with a single (0.1 %) patient among a total of 995 patients included into the international ADVANCE registry.11 These patients were exclusively undergoing TF TAVI, using the self-expandable Medtronic/CoreValve prosthesis.11
Single-center experiences involving smaller numbers of patients have suggested higher ECS rates of up to 2.8 % or even 4.9 %.12,13 These numbers may in part be related to different definitions of ECS12 but also to the sites‚‘ TAVI experience/learning curve.14 Griese et al.12 reported that 20 (4.9 %) of 411 patients undergoing TAVI between 2009 and 2012 at their institution required ECS, including four patients who switched to TA TAVI (n=1) or femoral cardiopulmonary bypass for cardiogenic/hemorrhagic shock (n=3), but no sternotomy. There was also a strikingly high rate of ventricular perforation with either the pacing lead or the stiff guidewire with failed transcutaneous puncture ultimately necessitating ECS.12
TAVI Complications Requiring Emergent Cardiac Surgery
Complications that occur during TAVI and require ECS include ventricular injury with tamponade (e.g. right or left ventricular perforation due to pacer lead or stiff guide wire), injury to the ascending aorta (e.g. perforation, dissection), rupture of the device landing zone (i.e. annular rupture), coronary obstruction, severe (para-)valvular regurgitation, and prosthesis embolization/migration into the left ventricle or aorta.7 The risk for these complications is generally low, ranging between 0.2 % and 1.0 % (see Table 1). In the German TAVI registry, which included 1,975 patients between 2009 and 2011, leading causes for ECS during TAVI were aortic injury (21 % of ECS cases), prosthesis embolization (21 %), and myocardial perforation (17 %).7 Similar distributions have been reported from the US TAVI registry, which included prosthesis embolization (23 % of ECS cases), aortic injury (13 %), and myocardial perforation (12 %), but also annular rupture (14 %) among the most frequent ECS causes.9 In the SOURCE registry of 2,307 patients, again prosthesis embolization (33 % of ECS cases) and aortic injury (26 %) were the main causes for ECS.10
Outcomes after Emergent Cardiac Surgery
Patients currently selected for TAVI are usually elderly, co-morbid, and fragile patients deemed either inoperable or at least high risk for elective open heart surgery. It is therefore not surprising that mortality of emergency heart surgery for complications occurring acutely during the TAVI procedure is high. In fact, mortality among patients requiring ECS during TAVI may be as high as 67 %, thus being approximately ninefold higher than in patients undergoing uncomplicated TAVI.6 Other studies reported somewhat better outcomes after ECS, but the 30-day mortality rates still varied between 45 % and 52 %.7,10,12
It has been suggested that the severity of the specific TAVI complication that necessitates ECS has an effect on postoperative outcomes. In the German TAVI registry, the highest mortality was observed in patients undergoing ECS for aortic perforation or dissection: four (80 %) out of five patients died despite ECS.7 High mortality rates after ECS were also reported for annular rupture (50–100 %) and cardiac tamponade (50–100 %).7,10,15,16 By contrast, outcomes of patients with severe aortic regurgitation undergoing ECS appeared to be better: postoperative mortality ranged between 0 % and 33 %.7,10 As patients with severe aortic regurgitation are usually hemodynamically more stable than patients with overt annular rupture and tamponade, it may be speculated that not only the complexity of the complication, but also the hemodynamic status of the patient allowing for example more urgent/semi-elective instead of bail-out emergency surgery has an impact on postoperative outcomes. The analysis of Seiffert et al.13 further suggests that patients with a lower baseline risk (i.e. lower EuroSCORE) may have better outcomes even after ECS for TAVI complications.14
Conclusion and Glimpse into the Future
ECS is currently required in approximately 1 % of patients undergoing TAVI and may be more frequent among those undergoing TA TAVI procedures. The Medtronic/CoreValve experience, which already excludes prosthesis embolization and annular rupture, suggests that the risk for ECS can be reduced to 0.1 % of TAVI patients.11 Currently, leading causes for ECS during TAVI are aortic injury, prosthesis embolization, and myocardial perforation, as well as annular rupture. Even in centers with an on-site cardiac surgery department and thus short reaction times in such bail-out situations, postoperative mortality of ECS is high (45–67 %), owing to the comorbid and fragile health status of inoperable or high-risk patient cohort currently selected for TAVI instead of open surgery. Therefore, minimizing ECS risk is the best way to improve outcomes.
It may be anticipated from the historical development of percutaneous coronary intervention that the need for ECS during TAVI may similarly decrease in the near future. Technical improvements in valve design such as the development of retrievable, repositionable TAVI devices may prevent prosthesis embolization and thus the need for ECS. Development of dedicated, pre-shaped stiff TAVI guidewires may reduce the risk for ventricular perforation. Better pre-procedural planning by routine use of contrast-enhanced computed tomography allows for better positioning, thus avoiding coronary obstruction and minimizing the risk for oversizing-induced annular rupture as well as paravalvular regurgitation by better prosthesis size selection. Paravalvular leak is further addressed by technical developments in valve design. The increasing tendency to avoid balloon valvuloplasty before valve implantation may reduce annular rupture. Similarly, miniaturization and improved flexibility of the delivery systems may help to reduce ECS for complications such as aortic perforation or dissection. Increasing operator experience and growing confidence in the procedure will further reduce the risk for ECS.
The 2012 valvular heart disease guidelines of the European Society of Cardiology mandate that TAVI should only be performed in hospitals with both cardiology and cardiac surgery department on-site. The requirement of an on-site cardiac surgery department as a prerequisite for TAVI has been approved with the highest level of recommendation (class 1); however, based on expert consensus only (level of evidence C). Scientific data to support this recommendation do not exist. Since 2012, TAVI has rapidly evolved and has made substantial technical (e.g., 14 F Sapien 3 prosthesis) as well as procedural (i.e., growing operator experience and confidence) progress to become a routine procedure, which is highly beneficial for patients at high risk for open surgery. In 2013, >10,000 TAVI procedures were performed in Germany, which was more than the number of isolated AVR. The risk for severe complications necessitating ECS during TAVI is low and will further decrease. If required, outcomes of ECS are bleak, mostly due to the risk profile of the patients currently considered for TAVI. Therefore ECS does thus not really serve as a valid safety net. Avoidance of complications by experienced operators appears to be more appropriate to improve outcomes of TAVI.