Coronary calcification represents a significant technical challenge in percutaneous coronary intervention (PCI). It is estimated that moderate or severe calcification is present in approximately one-third of patients undergoing PCI.1 During PCI, the presence of significant calcification can result in difficulties delivering equipment and increase the risk of stent underexpansion and malapposition as well as complications such as perforation and dissection.2 As a result, calcification is associated with an increased risk of repeat revascularisation, stent thrombosis and death.1,3 Coronary atherectomy devices, such as rotational and orbital atherectomy, have been shown to be effective in facilitating procedural success in calcified lesions.4,5 However, atherectomy devices may be unavailable in certain centres and may be undesirable in some clinical situations, such as bifurcation PCI requiring uninterrupted side-branch access. Fortunately, a number of balloon-assisted technologies are available to aid in the management of coronary calcification before stenting. These include non-compliant (NC) balloons, super high-pressure NC balloons, cutting balloons, scoring balloons and intravascular lithotripsy (IVL).
Non-compliant Balloons
In some circumstances, standard NC balloons can be used to adequately prepare calcified lesions prior to stent implantation. At higher pressures, NC balloons have a more predictable diameter compared with semi-compliant balloons. This partly avoids ineffective and non-uniform balloon expansion, known as ‘dog-boning’, which can result in vessel dissection and perforation. When used routinely for lesion predilation, NC balloons have been demonstrated to result in better stent expansion compared with semi-compliant balloons.6 Although NC balloons can be inflated to relatively high pressures (up to 20–24 atmospheres), standard NC balloons may still be vulnerable to dog-boning or rupture in heavily calcified vessels. As such, balloon expansion should be assessed using orthogonal views to ensure that adequate and uniform expansion has been achieved.
Super High-pressure Non-compliant Balloons
Super high-pressure NC balloons, such as the OPN balloon (SIS Medical), are double-layered balloons capable of expansion to super high pressures (e.g. 35 atmospheres in the case of the OPN balloon).7 Secco et al. retrospectively assessed the efficacy of OPN balloons in 91 patients with calcified lesions that could not be dilated with a regular NC balloon.7 Percent diameter stenosis was noted to be significantly lower after OPN ballooning compared with the NC balloon (41.1 ± 15.8 versus 20.2 ± 14.6; p<0.001).7 Prospective, randomised data are also available to support the use of super high-pressure NC balloons. The ISAR-CALC trial randomised 74 patients with severely calcified lesions with unsuccessful NC balloon pre-dilatation (defined as a <30% reduction in baseline diameter stenosis) to undergo lesion preparation with either a super high-pressure balloon or a scoring balloon.8 Compared with the scoring balloon, super high-pressure ballooning increased the minimum lumen diameter (2.83 ± 0.34 mm versus 2.65 ± 0.36 mm; p=0.03) and reduced the diameter stenosis (11.6 ± 4.8% versus 14.4 ± 5.6%; p=0.02).8 Strategy success, defined as target lesion residual stenosis <30%, Thrombolysis in Myocardial Infarction (TIMI) score 3 flow and no major adverse cardiac events achieved without the need for additional devices for lesion preparation, was also high in the super high-pressure balloon group at 91.9%. In one retrospective study of 326 patients with calcified coronary lesions undergoing PCI with OPN balloons, perforation was reported in 0.9%.9 Therefore, complications secondary to super high-pressure balloon inflation appear to be relatively uncommon. This safety is mainly dependent on the recommended avoidance of 1:1 reference vessel dilation but rather a 0.6 to 0.7 ratio, similar to that recommended for cutting balloons. Care should also be taken to inflate these balloons slowly (increased by 5 atmospheres every 10–20 seconds after 20 atmospheres) using a dedicated inflation device.
Given their bulkier twin-layer design, delivery of these balloons can be difficult. Therefore, larger, and more supportive guiding catheters, more supportive wires, such as the WIGGLE wire (Abbott), alternate wiring techniques, such as ‘buddy’ wiring, and other devices, such as guide extensions, may need to be considered when using this device. One additional consideration is that super-high-pressure balloon inflations may result in ‘fusion’ of the device with the coronary guidewire, which is more likely when using Asahi Intecc wires (i.e., SION Blue or PROWATER) because of the transition part with the coating where the balloon shaft internal layer (hypotube) becomes fused (or stuck). The consequent loss of wire position on device removal may be of significant consequence if dissection has occurred as a result of balloon inflation; therefore, an upfront buddy wire would provide a ‘safety net’. Super high-pressure balloons may also have little impact on nodular or eccentrically calcified plaques.
Cutting Balloons
Cutting balloons are NC balloons with three-to-four longitudinally arranged microsurgical atherotomes bonded to their surface.10 The atherotomes focus pressure over a smaller surface area, which can create incisions in calcified plaques.11 When using a cutting balloon, it is recommended that a balloon that approximates the reference vessel diameter be selected and the device should be inflated slowly (1 atmosphere/5 seconds). The REDUCE III study (n=521) compared the cutting balloon with balloon angioplasty prior to stenting with bare-metal stents.12 In this study, minimum lumen diameter was significantly higher in the cutting balloon group (2.65 ± 0.40 mm versus 2.52 ± 0.4 mm; p<0.01). Furthermore, rates of restenosis were significantly lower with cutting balloons (11.8 versus 19.6%; p<0.05), as was target lesion revascularisation (9.6 versus 15.3%; p<0.05). Importantly, the outcomes observed in the cutting balloon group were conveyed almost exclusively by the subgroup of patients who had their procedure guided by intravascular ultrasound (IVUS), highlighting the importance of imaging in the effective use of cutting balloons. The COPS trial was a prospective, randomised, multicentre trial which enrolled patients with significantly calcified lesions (≥100° arc) as assessed by IVUS.13 A total of 87 patients were randomly assigned to undergo predilation with either a WOLVERINE cutting balloon (Boston Scientific) or an NC balloon prior to new-generation drug-eluting stent insertion. Of note, operators were encouraged to undersize cutting balloons by 0.5 mm to reduce the risk of vessel perforation. Final minimum stented area (MSA) at the calcified segment was found to be significantly higher in the cutting balloon group compared with the NC balloon group (8.1 ± 2 versus 7.3 ± 2.1; p=0.035).13
Cutting balloons may also serve as a useful adjunct to rotational atherectomy (RA). One single-arm prospective study (n=110) compared a strategy of RA followed by cutting-balloon inflation (‘rota-cut’) and used the modified balloon (including cutting balloons and sculpting balloons) and RA cohorts from the PREPARE-CALC study as an historical comparison.14.15 Acute lumen gain was significantly higher with a rota-cut strategy compared with RA or modified balloon alone (1.92 ± 0.45 mm versus 1.74 ± 0.45 mm with modified balloon versus 1.70 ± 0.42 mm with RA; p=0.001 and p<0.001, respectively).14 Stent expansion was found to be similar between the groups (75.1 ± 13.8% versus 73.5 ± 13.3% with the modified balloon versus 73.1 ± 12.2% with RA; p=0.19 and p=0.39, respectively) but MSA was noted to be significantly higher with a rota-cut strategy (7.1 ± 2.2 mm2 versus 6.1 ± 1.7 mm2 with the modified balloon versus 6.2 ± 1.9 mm2 with RA; p=0.003 and p=0.004, respectively). Furthermore, there were no significant differences in complications such as perforation, dissection, slow/no flow, or in-hospital death, MI or repeat revascularisation.
Historically, one notable issue associated with cutting balloons was their deliverability. However, developments in cutting balloon technology including reduced entry profiles, more deliverable catheters, reduced balloon size and improved distal tip flexibility appear to have improved this significantly and have resulted in lower rates of device failure.13,16 Nonetheless, adjunctive strategies may still be required to facilitate device delivery. Furthermore, as with super high-pressure balloons, cutting balloons are unlikely to have a significant effect on eccentric or nodular calcification.
Scoring Balloons
Scoring balloons, such as the AngioSculpt (Philips), are semi-compliant balloons encased within three-to-four helically-arranged nitinol struts.16 When inflated, these struts score the lumen surface and facilitate lesion dilatation. It is recommended that a scoring balloon one size below the reference vessel diameter be used (e.g. a 2.5 mm device for a 3.0 mm vessel) with slow (2 atmospheres/10–15 seconds) inflations. Data from an observational study comparing direct stenting (n=145), lesion preparation with a semi-compliant balloon (n=117) and lesion preparation with a scoring balloon (n=37) demonstrated significantly higher MSA with the scoring balloon strategy (6.0 ± 1.7 mm2 for direct stenting versus 5.9 ± 1.6 mm2 for semi-compliant balloon versus 6.8 ± 1.5 mm2 for scoring balloon; p=0.02).17 Another observational study compared the use of a scoring balloon (n=146) with a cutting balloon (n=173) in calcified lesions with the primary aim of assessing device deliverability.18 Stent delivery success rates were found to be significantly higher after the cutting balloon than the scoring balloon (90.8 versus 79.5%; p=0.006). In addition, even despite the smaller pre-procedural minimum lumen area (MLA), longer lesion length and more circumferential calcification recorded in the cutting balloon group, there was no significant difference in cross-sectional area gain post-stenting.
Randomised data assessing the efficacy of the scoring balloon in preparing calcified vessels are also available. In the PREPARE-CALC study, 200 patients with severely calcified lesions were randomly assigned to undergo lesion preparation with either a modified balloon (cutting balloon or scoring balloon) or RA.15 The two primary endpoints were strategy success (defined as successful stent delivery and expansion with attainment of <20% in-stent residual stenosis in the presence of TIMI 3 flow without crossover or stent failure; powered for superiority) and in-stent late lumen loss at 9 months (powered for noninferiority). Strategy success was significantly lower in the modified balloon group compared with the RA group (81 versus 98%; RR of failure with a modified balloon-based versus RA-based strategy, 9.5; 95% CI [2.3–39.7]; p=0.0001). However, the modified balloon strategy was non-inferior to the RA strategy regarding late lumen loss. Importantly, it should be noted that most of the modified balloon group underwent preparation with a scoring balloon (96.7%) with only 3.3% receiving a cutting balloon. Regarding safety, there were no significant differences in the incidence of perforation, dissection, slow/no flow or in-hospital death, MI or repeat revascularisation.
More recently, Rheude et al. published a pooled patient-level analysis of patients (n=200) from the ISAR-CALC and the PREPARE-CALC trials who underwent imaging with optical coherence tomography (OCT) and compared patients who underwent treatment with RA (n=63), modified balloon (n=103) and super high-pressure balloons (n=34).19 Stent expansion (defined as MSA/reference lumen area × 100) was not significantly different between the three groups (73.2 ± 11.6% for RA versus 70.8 ± 13.6% for modified balloon versus 71.8 ± 12.2% for super high-pressure balloon; p=0.49). Notably, compared with RA and modified balloons, a super high-pressure balloon strategy was associated with less stent eccentricity (p=0.08). Strategy success was noted to be significantly higher with RA compared with modified balloons (100 versus 86.4%; p=0.002) and numerically, but not significantly, more frequent with RA compared with super high-pressure balloons (100 versus 91.2%; p=0.08). Although numbers were low, there was also no significant difference in coronary perforation, stent thrombosis, dissection or major adverse cardiovascular events (MACE).
NC scoring balloons are also available. These include the Scoreflex (OrbusNeich), which uses a short rapid-exchange tip and an integral wire outside of the balloon, which allows the integral wire and the guidewire to act as scoring elements. A single arm prospective study of 200 patients, of whom 36.6% had moderate-to-severe target lesion calcification, reported favourable results.20 Procedural success – defined as successful delivery, inflation, and deflation of the device; the absence of perforation, flow-limiting dissection or reduction in TIMI flow grade; and final achievement of TIMI 3 flow – was found to be high, at 93.5%.
Like super high-pressure balloons and cutting balloons, scoring balloons may also require adjunctive techniques and equipment to facilitate delivery and are limited in their effect on eccentric and nodular calcium.
Intravascular Lithotripsy
The IVL balloon, such as the Shockwave IVL (Shockwave Medical), is among the newest balloon-based calcium modification technologies. IVL balloons are usually sized 1:1 with the reference vessel and are advanced over an 0.014 inch guidewire. Balloons are inflated to low atmospheric pressure (4 atmospheres) to achieve vessel wall contact but minimise barotrauma. IVL uses low levels of electrical energy to produce vapour bubbles, which rapidly expand and produce acoustic pressure waves that radiate circumferentially from the balloon.21 These pressure waves propagate through soft tissue with negligible effect but produce compressive stress on areas of calcification, which may produce fractures in both superficial and deeper layers.21
The Disrupt CAD I study was a prospective, multicentre study which described the first in-human use IVL (n=60) and demonstrated its feasibility with encouraging initial success and complication rates.22 This was followed by the larger single-arm, prospective Disrupt CAD II study (n=120) which enrolled patients with severe coronary calcification (based on angiographic assessment) undergoing PCI.23 High levels of clinical success (94.2%) were achieved with relatively low rates of 30-day MACE (7.6%). Three dissections were noted but there were no instances of perforation, slow flow or no reflow. In the 48 patients who underwent OCT pre-stenting and 47 who underwent OCT post-stenting, IVL was effective in increasing lumen area (2.33 ± 1.35 to 6.10 ± 2.17 mm2; p<0.0001) and decreasing calcium angle (175.8 ± 96.9 to 127.1 ± 97.6º; p=0.055)
The Disrupt CAD III study (n=384) has provided longer-term follow-up data in patients with severe calcification undergoing PCI for stable or unstable angina undergoing IVL.24 In this single-arm prospective study, 12-month MACE was reported in 13.8%, which was considered relatively low when compared with the 16.9% rate observed in the ORBIT II study using orbital atherectomy.25 Low rates of perforation (0.3%) and flow-limiting dissections (0.3%) were reported, with no instances of slow flow/no reflow. The 2-year outcome data for 347 individuals demonstrated a target lesion failure rate of 16.1%.26
IVL may provide an advantage over other balloon-based and atherectomy devices in managing eccentric and nodular calcium. In calcified vessels, high-pressure balloon inflations can often disrupt or dissect healthy intima without modifying calcium.27 In theory, cutting balloons and scoring balloons are also similarly limited, while atherectomy devices may also be inadequate when wire bias is present. On the other hand, the mechanism of calcium modification during IVL treatment may lend itself to modifying eccentric calcium. One patient-level pooled analysis of 180 patients enrolled in the Disrupt CAD I and CAD II studies provides some evidence for this.28 In this study, 47 eccentrically calcified lesions were compared with 133 concentrically calcified lesions. The study found no difference in clinical success (residual stenosis <50% and no in-hospital MACE) between lesion types (93.6 versus 93.2%; p=1.0) and no difference in residual stenosis (8.6 ± 9.8% in eccentric and 10.0 ± 9.0% in concentric; p=0.56). A similar patient-level pooled analysis of 248 calcified lesions from the Disrupt CAD studies also demonstrated the efficacy of IVL in treating nodular calcium.29 In this study, 21.8% of lesions undergoing lesion preparation with IVL were noted to have a calcium nodule identified by OCT. Post-PCI, there were no significant differences in MLA (6.5 mm2 for nodular lesions versus 6.1 mm2 for non-nodular lesions), MSA (6.3 mm2 for nodular lesions versus 6.0 mm2 for non-nodular lesions) and mean stent area (8.3 mm2 for nodular lesions versus 7.9 mm2 for non-nodular lesions) at the site of pre-IVL MLA, with no major complications reported.
IVL has been used in the treatment of under expanded stents in calcified vessels. Registry data suggest that this can be performed safely with significant increases in both stent and lumen dimensions.30 However, concerns have been raised that IVL may disrupt the stent polymers and antiproliferative agents integral to drug-eluting stent function and, thus, may promote in-stent restenosis. An ex vivo study of an everolimus-eluting fluoropolymer-coated drug-eluting stent demonstrated microscopic cracks, tears and detachments of the fluoropolymer coating after delivering 80 shocks with an IVL balloon.31 However, the overall integrity of the fluoropolymer remained preserved and the degree of disruption was not thought to be significant enough to interfere with antiproliferative drug delivery. Moreover, the study reconstructed a situation in which an NC balloon was passed forcibly through an under expanded stent, which showed similar but more extensive fluoropolymer disruptions.
Despite promising early evidence, the most significant limitation of IVL, as with other balloon-based calcium modification techniques, is its deliverability. While this can be overcome with adjunctive techniques and devices in many circumstances, atherectomy or laser devices may still be required in balloon-uncrossable lesions. The combination of atherectomy and IVL, which may provide complimentary modification of both superficial and deep calcium respectively, has also been proposed as a means of achieving superior lesion preparation.32,33
Conclusion
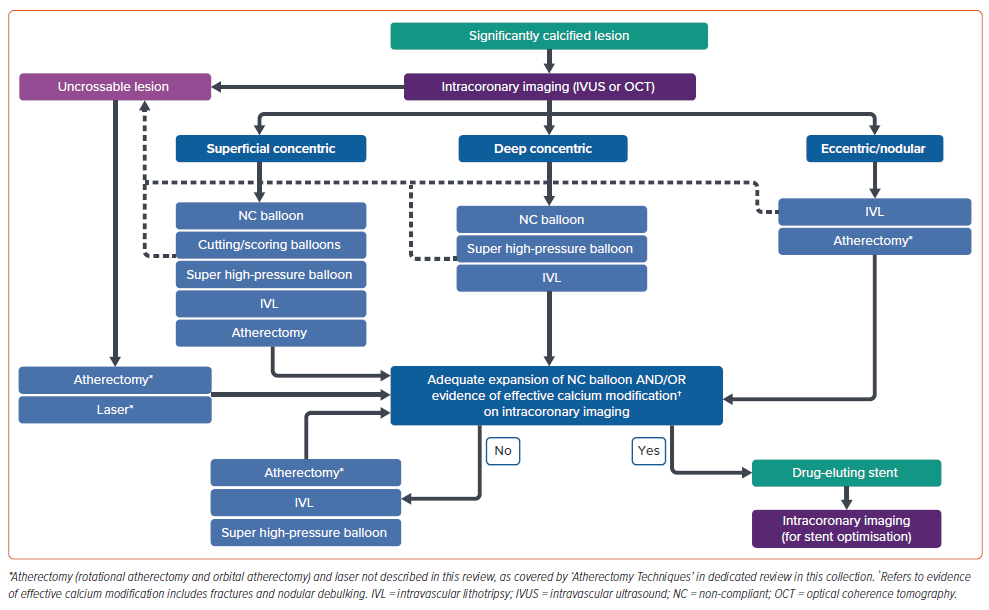
Adequate lesion preparation is crucial in the successful management of calcified coronary lesions. Balloon-based techniques can be safe and effective tools in the management of significant calcification. However, current data supporting the use of balloon-based techniques for calcium modification focus primarily on intra-procedural and short-term clinical outcomes, with data supporting longer-term clinical outcomes lacking. Furthermore, although these technologies have progressed significantly in recent years, deliverability of these devices remains a significant issue. Despite these limitations, in addition to intracoronary imaging and atherectomy and laser devices (covered in separate dedicated articles of this themed section of the journal), they continue to be an important component of contemporary calcium management algorithms (Figure 1).