Many profound physiological changes take place in the cardiovascular system during pregnancy to meet the increased metabolic demands of both the mother and foetus. Such changes include an increase in circulating blood volume and cardiac output and decreases in systemic vascular resistance, blood pressure and hypercoagulation.1,2 The increase in circulatory burden during pregnancy and the postpartum period can unmask pre-existing undiagnosed cardiac disease, cause the deterioration of known heart disease or lead to the development of a new one.1
Cardiovascular disease in pregnancy is an increasingly important cause of maternal morbidity and mortality.3 The latest report from the Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK (MBRRACE-UK) showed that cardiac disease is the leading indirect cause of maternal deaths up to 6 weeks after the postpartum period.4 This report is supported by the Confidential Enquiries into Maternal and Child Health (CEMACH) finding that in the UK, the overall rate of mortality secondary to cardiac disease has risen from 7.3 per million births in the 1982–1984 triennium to 22.7 per million births in the 2003–2005 triennium.5 Despite this increase, data from the MBRRACE-UK report showed that 90% of pregnant women who died between 2014 and 2016 did not have a pre-existing cardiac condition.4 It appears that the major part of this increase is attributable to acquired cardiac disease, with one-third of these deaths being secondary to acute MI (AMI) or ischaemic heart disease.4 Understandably, there is concern among cardiologists and obstetricians regarding the treatment of AMI in pregnancy, especially percutaneous coronary intervention (PCI). This article will focus on AMI in pregnancy and PCI in detail.
Acute MI in Pregnancy
AMI in pregnancy leads to poor maternal and foetal outcomes, and the mortality rate is twice as high in cases where AMI occurs during the peripartum period.6 The incidence of coronary artery disease (CAD) in women of child-bearing age is currently low and AMI is quite uncommon in this population (3–100 per 100,000 deliveries).7 Interestingly, a large UK-based study recently demonstrated that prior hypertensive disorders of pregnancy, such as gestational hypertension and eclampsia, were associated with increased arterial stiffness and a long-term risk of a range of cardiovascular diseases, including CAD.8 Thus, there is a possibility for the incidence of CAD in women of child-bearing age to increase in future with the increasing incidence of hypertensive disorders of pregnancy.8
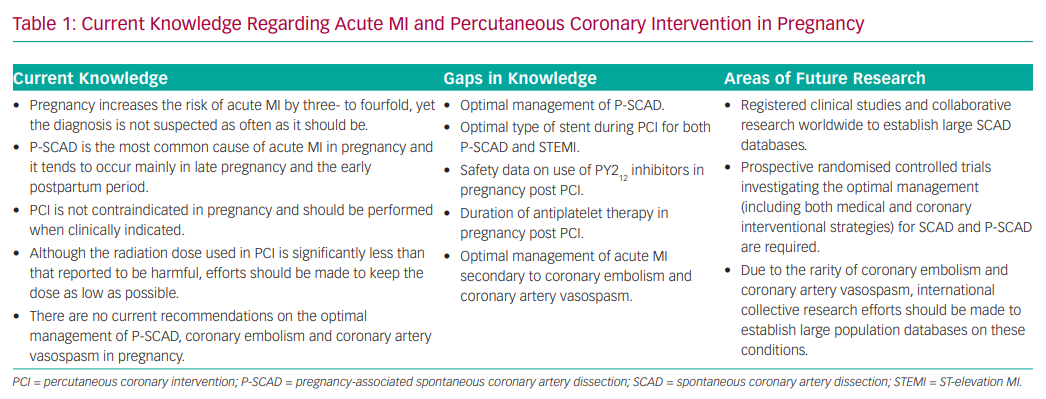
Despite the low CAD rate, pregnancy increases the risk of AMI by three- to fourfold compared to non-pregnant women of similar age (Table 1).9 This risk is age-related, being 30 times higher for women >40 years of age compared with women aged <20 years.10
Over the past two decades, there has been an increase in the use of fertility therapy, such as in vitro fertilisation, particularly among older women.11 Fertility therapy often involves repeated cycles of high-dose hormonal stimulation protocols and superovulation itself is pro-thrombotic;12,13 therefore, it is plausible that in vitro fertilisation and other fertility therapy techniques contribute to cardiovascular risk.12 However, a recent systematic review and meta-analysis reported no increased risk of developing an acute cardiac event following fertility therapy.12 This finding is supported by a large population-based Canadian study involving 6,979 women, where the authors concluded that successful fertility therapy was not associated with an increased risk of cardiovascular disease in later life.11
Overall, the incidence of AMI is higher in multigravidas and during the third trimester.6 Pregnant women with AMI during the postpartum period tend to be younger than those experiencing AMI during the antepartum or peripartum periods.14 Aside from traditional cardiovascular risk factors, other risk factors specific to pregnancy include pre-eclampsia, the presence of prosthetic valves, anaemia and thrombophilia.15,16 Despite the increased risk of AMI in pregnancy, one US-based study found that of 859 patients presenting with AMI during pregnancy and the postpartum period, only 45% had undergone cardiac catheterisation.17 The authors highlighted that the diagnosis of AMI is not suspected as often as it should be and that there is a general reluctance of physicians to intervene.17
MI with Obstructive Coronary Arteries
ST-elevation in MI in Pregnancy
ST-elevation MI (STEMI) in pregnant women involves the anterior wall in 70–80% of cases.18 In more than half of cases, reduction of left ventricular ejection fraction to <40% was observed, leading to a high incidence of heart failure, cardiogenic shock and ventricular arrhythmias.18 Diagnostic criteria are the same as for patients who are not pregnant and are based on clinical symptoms, ECG changes and an increase in troponin levels.19,20 It should be noted that ST elevation is not seen in normal pregnancy and warrants urgent attention.19 STEMI in pregnant women should be managed in the same way as in non-pregnant women. Given the high mortality associated with STEMI in pregnancy, the European Society of Cardiology (ESC) recommends primary PCI as the preferred reperfusion therapy.13
Non-ST-elevated MI in Pregnancy
Similarly to STEMI, there are no differences in diagnostic criteria between pregnant and non-pregnant patients presenting with non-ST-elevation MI (NSTEMI). It is important to note that ST segment depression and T-wave inversion can be a normal variant seen in pregnancy.19
Both the American Heart Association and ESC guidelines recommend that myocardial revascularisation with PCI be reserved for pregnant women with NSTEMI who are unstable or present with serious complications unresponsive to medical therapy.13,21
MI with Non-obstructive Coronary Arteries
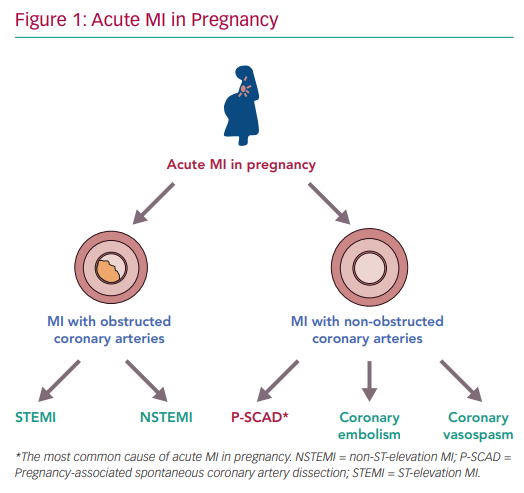
In pregnancy, causes of MI with non-obstructive coronary arteries (MINOCA) include spontaneous coronary artery dissection (SCAD), coronary embolism and severe coronary artery vasospasm (Figure 1).22 While pregnancy-associated SCAD (P-SCAD) is more common, there are a few case reports of AMI secondary to coronary embolism and coronary artery vasospasm.22–25
P-SCAD
In the general population, the majority of AMI occurs as a result of coronary atherosclerosis, typically leading to STEMI or NSTEMI. In pregnancy, SCAD is the most common cause of AMI and tends to occur mainly in late pregnancy or during the early postpartum period.7,18,20 Although previously considered rare, it has recently become clear that SCAD is an important and underdiagnosed cause of AMI in women.26,27 P-SCAD makes up <10% of the total number of SCAD cases.26,27
In the past, SCAD was frequently reported as a disorder mostly affecting women with no risk factors for cardiovascular disease. However, a recent study has demonstrated that conventional cardiovascular risk factors, such as hypertension, dyslipidaemia and smoking, are not uncommon in patients presenting with SCAD.28
P-SCAD is most frequent during the first postpartum month, but cases have been reported during early pregnancy and up to 18 months postpartum.29,30 It has been suggested that this might be related to cardiac stress secondary to rapid post-delivery uterine contraction and the return of a copious volume of blood to the systemic circulation.31,32 Most patients tend to have a history of multiple pregnancies.29,32 Although the association is unclear, there is a potential link with arterial degeneration, which could be compounded by multiple pregnancies.33
Studies have shown that SCAD is not benign and can have complications such as life-threatening ventricular arrhythmias and sudden cardiac death.30 P-SCAD patients can have a more severe clinical presentation, such as acute heart failure and multivessel dissections, than patients with non-pregnancy-associated SCAD.32 Additionally, it has significant reported recurrence of ~10% at 3-year follow-up and major adverse cardiovascular event rates.34,35
In a recent Canadian prospective cohort study of 236 SCAD patients, the rate of recurrent MI was 19.1%, recurrent SCAD was 12.7%, stroke or transient ischaemic attack was 1.3% and mortality was 1.7% at a median 2.3-year follow-up.36 Thus, women of child-bearing age with a history of SCAD should be carefully counselled regarding the risk of recurrent events.
Pathophysiology
It is rare for patients with SCAD to have recognised hereditary connective tissue disorders, such as Marfan syndrome and vascular Ehlers-Danlos syndrome.34,37 With SCAD, there is sudden disruption of the coronary artery wall, resulting in separation of the inner intimal lining from the outer vessel wall, leading to an intramural haematoma (Figure 2), or rupture of the vasa vasorum, leading to intramural haematoma.30 This can cause luminal compression and obstruction and, if the haematoma expands, can cause propagation of the dissection plane.30,38 Typically, patients with SCAD have fragile arterial walls with no atheroma or calcification to limit the propagation of dissection, which means the dissections tend to be more extensive.30
There have been several proposals in the literature regarding the pathophysiology of P-SCAD. Although the exact mechanism is undetermined, the proposals focus on hormonal and haemodynamic changes as possible causes. These changes include excess progesterone leading to structural weakening of the vessel wall and consecutive shearing stress secondary to increased cardiac output and circulatory volume.18,29,39
Clinical Presentation and Diagnosis
The clinical presentation of P-SCAD is dependent on the extent and rate of dissection as well as the degree of myocardial ischaemia.40 Patients can present with STEMI, but may also have more serious presentations, such as cardiogenic shock or pericardial tamponade.29 Tamponade could result from direct extension of the dissection into the pericardial space, rupture of infarcted myocardium or from post-infarction pericarditis.41 Clinical symptoms of P-SCAD include chest pain, dyspnoea, diaphoresis, nausea, vomiting and a ‘popping’ or ‘clicking’ sensation in the chest.29,32 Most patients have elevated troponin levels, although it has been observed that there is a wide variation in the rise in troponin I.42
Coronary angiography is the first-line diagnostic imaging method in SCAD due to its wide availability.43,44 It is of vital importance that extra care is taken, as there is a risk of iatrogenic extension of the dissection.45 When there is diagnostic uncertainty, intracoronary imaging using optical coherence tomography and intravascular ultrasound can allow detailed visualisation of the arterial wall.43,44
Another study has shown that in P-SCAD there are more frequent findings of left ventricular ejection fraction ≤35%, and patients are more likely to experience SCAD affecting the left main or multiple vessels.32 There are multiple reports that the left anterior descending artery is the most frequently affected vessel.17,29,46 The haemodynamic and anatomical differences between the right and left coronary arteries could explain this finding. For instance, the left anterior descending artery is subjected to increased torsion force during the cardiac cycle due to a higher number of branches than the right coronary artery.7,47
Management
Optimal management of P-SCAD is controversial. Generally, the ‘conservative if possible’ approach is preferred; both recent European and US consensus statements emphasise a preference for a conservative approach.43,44 In the majority of cases, arteries affected by SCAD heal spontaneously and studies have suggested that revascularisation is associated with a high failure rate.38,48,49 Additionally, PCI for SCAD has an increased risk of extending the dissection and requiring emergency surgery.29,38 However, the lack of randomised controlled trials means there is no specific recommendation; the decision ultimately depends on the clinical presentation, extent of coronary artery dissection and size of myocardium at risk. Nevertheless, in patients with ongoing or recurrent ischaemia, haemodynamic instability or isolated left main dissection, it has been suggested that PCI should be performed if the anatomy is suitable.50
Coronary Embolism
Hypercoagulable states, such as pregnancy, increase the risk of embolic disease.51 Thus, despite its rarity, coronary embolism is an important condition to be aware of as a cause of MINOCA during pregnancy. ST segment elevation can be seen on ECG in the majority of patients presenting with coronary embolism.52 The diagnosis is challenging, but clinical suspicion should be increased in patients with predisposing conditions, such as AF, right-to-left cardiac shunt, prosthetic heart valves and antiphospholipid syndrome as well as a low likelihood of CAD.53 Typically, coronary embolism affects the left coronary system as the left main artery is larger than the right coronary artery and receives a greater proportion of blood flow, making it more likely to receive emboli.54
There are three types of coronary embolism: direct, paradoxical and iatrogenic.55 Direct coronary emboli typically arise from the left atrial appendage, left ventricle or aortic and mitral valves.55 Pregnant women with mechanical prosthetic valves are particularly vulnerable as the valves themselves are thrombogenic and it is difficult to achieve adequate anticoagulation.25 This is evident in the case reports of AMI in pregnancy secondary to thromboembolism originating from prosthetic valves.23,25 Paradoxical emboli originate from the venous system and usually pass through a patent foramen ovale, an atrial septal defect or pulmonary arteriovenous malformations.
Pregnancy is associated with a fourfold increased risk of venous thromboembolism, but the incidence of paradoxical coronary embolism is unknown.24,55 One case has reported on coronary embolism in pregnancy secondary to a paradoxical embolus, but the patient was a factor V Leiden carrier.24 With the increased use of coronary angiograms, valvuloplasty and other invasive coronary interventions, iatrogenic coronary embolism is currently the most common cause of embolism in the coronary arteries.52,54 Possible mechanisms for this may be the formation of clots in catheters, accidental introduction of air during invasive procedures and, on rare occasions, embolisation of friable calcific valvular material from the aortic valve into the coronary arteries during such procedures.52,55
At present, there is no consensus regarding the optimal management of AMI secondary to coronary embolism due to its rarity. Various attempts to treat the condition have been described in numerous case reports using intracoronary thrombolysis, aspiration catheter and ballooning and/or stenting, with varying success.25 Large population-based studies in this area are required to obtain further knowledge (Table 1).
Coronary Artery Vasospasm
AMI secondary to coronary artery vasospasm in pregnancy is very rare.22 One case has been reported in the literature where a woman in her 38th week of pregnancy presented with sudden severe substernal central chest pain and ST-elevation.22
According to the ESC consensus document on vasospastic angina, coronary artery spasm is defined as transient total or subtotal coronary artery occlusion (>90% constriction) with angina and ischaemic ECG changes either spontaneously or in response to a provocative stimulus, such as acetylcholine and hyperventilation.56 Diagnosis of coronary artery spasm itself is challenging and often requires provocative testing with intracoronary acetylcholine during invasive coronary angiography, where >90% vasoconstriction is the angiographic threshold to diagnose inducible spasm.56 Smoking is a risk factor and East Asians could potentially be predisposed to coronary vasospasm.56,57 In cases of recent STEMI requiring reperfusion therapy, it has been reported that Japanese patients had hyper-reactive vessels compared to white patients.57
One of the proposed mechanisms for coronary vasospasm is endothelial dysfunction, as it promotes coronary vasoconstriction.15 In pregnancy, pre-eclampsia is a strong risk factor as it causes systemic endothelial dysfunction due to imbalance in the secretion of endothelin and thromboxane.16 Other suggested causes of coronary vasospasm in pregnancy include enhanced vascular reactivity to angiotensin II and noradrenaline, renin release and angiotensin production due to decreased uterine perfusion in the supine position, and the use of ergot derivatives to control pregnancy-related haemorrhage.9,58–61
AMI can result from significant occlusions secondary to prolonged and intense coronary vasospasm or if there is coronary vasospasm with superimposed thrombosis.52,62 Vasodilators, such as calcium channel blockers and nitroglycerin, are used to treat it, but little has been published regarding diagnostic strategies and therapeutics (Table 1).22
Percutaneous Coronary Intervention in Pregnancy
Any procedure involving radiation can cause concern for both the healthcare professional and the pregnant woman, and this can influence decision-making regarding PCI. Pregnancy is not a contraindication for PCI; as a life-saving procedure it should be performed when necessary. Management should be determined by a multidisciplinary team consisting of cardiologists, obstetricians, anaesthesiologists and neonatologists, and patients should be treated in an intensive care unit that can provide meticulous maternal monitoring and obstetric care.9,13
Based on ESC guidelines, the best time to perform any PCI procedure is after the fourth month during the second trimester.13 The reasoning behind this is mainly due to the completion of foetal organogenesis, inactive state of the foetal thyroid and the small uterine volume at this time, allowing a greater distance between the foetus and chest than in the later months of pregnancy.
Arterial Access
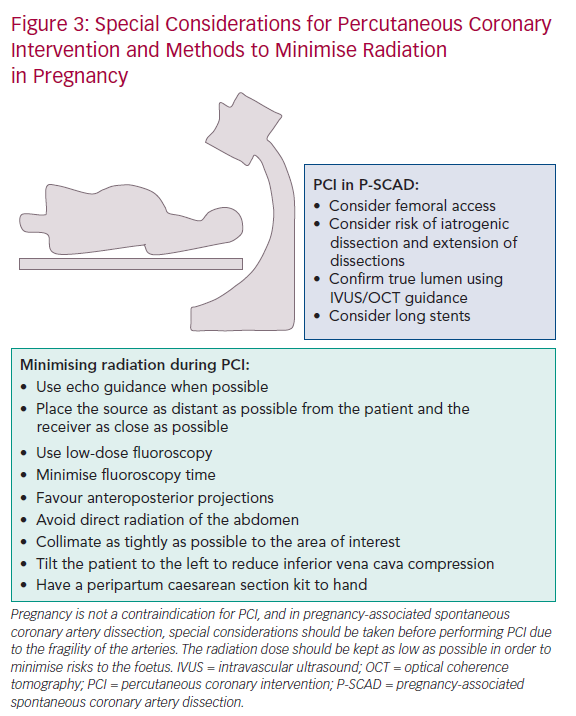
The latest ESC guidelines recommend that the radial approach by an experienced operator is preferable.13 Femoral artery entry allows direct pelvic radiation, which could theoretically increase the dose absorbed by the foetus. Additionally, there are more technical challenges associated with the femoral approach, due to the presence of the enlarged uterus and difficulties in positioning the woman.19 A recent meta-analysis of randomised controlled trials on radial versus femoral access for PCI in STEMI patients showed that the radial approach is favourable as it is associated with decreased bleeding complications, reduced length of hospital stay and improved patient comfort.63
Ionising Radiation Exposure
Concern regarding PCI in pregnancy usually stems from the risks associated with foetal exposure to ionising radiation. The dose of radiation absorbed and stage of pregnancy can determine the potential risks that ionising radiation exposure poses to the foetus.13,64 The highest levels of risk are during organogenesis and the early foetal period; the risk decreases as pregnancy progresses from the second trimester.65,66 ESC guidelines recommend that during cardiac catheterisation, the mean radiation the unshielded abdomen is exposed to should be 1.5 mGy, where <20% reaches the foetus.13 This dose is far lower than doses reported to be associated with foetal malformation, growth restriction or abortion (>50 mGy).67,68
Use of Iodinated Contrast Agents During Pregnancy
Iodinated contrast material can cross the placenta and enter the foetus;69 however, it has not been reported to cause teratogenic effects.70 Another concern is the potential risk of foetal congenital hypothyroidism.71 A 2010 study showed that there is no serious risk of neonatal hypothyroidism secondary to iodinated contrast agents, but there is a gap in the literature regarding this.72
Methods to Minimise Radiation
Although the mother can be reassured that the risk of radiation to the foetus is small, procedures should follow the radiation dose to be ‘as low as reasonably achievable’ principle (Figure 3). In order to achieve this, the ESC recommends the following manoeuvres:
- Use echo guidance when possible.
- Place the source as distant as possible from the patient and the receiver as close as possible.
- Use only low-dose fluoroscopy.
- Favour anteroposterior projections.
- Avoid direct radiation of the abdominal region.
- Collimate as tightly as possible to the area of interest.
- Minimise fluoroscopy time.
- Use an experienced cardiologist.13
Abdominal shielding is of limited benefit as the dose absorbed by the foetus is the result of internal scatter from thoracic tissues rather than direct foetal irradiation from the X-ray beam.13,73
Other techniques suggested to minimise radiation to the patient include using a lower frame rate (7.5 frames/s), using wedge filters, changing the projection frequently to distribute radiation and opening the iris on the television camera, which allows a lower increase in beam intensity.74,75
Stent Selection
The type of stent deployed requires careful consideration, as this affects the duration of antiplatelet therapy after implantation.19 Bare-metal stents are commonly employed for STEMI in pregnancy, especially in the third trimester.19 This allows for interruption of dual antiplatelet therapy at the time of delivery, reducing potential bleeding complications.
New-generation drug-eluting stents are recommended for patients with STEMI who are not pregnant in the 2017 AMI STEMI guidelines, because trials have demonstrated that they are superior to bare-metal stents in patients with AMI, even with the use of short-duration dual antiplatelet therapy.76–78
Special Considerations in P-SCAD
Typically, the arteries in SCAD are prone to iatrogenic dissections and extension of dissections during PCI due to the fragility of the arterial walls.79 It is vital that PCI is undertaken cautiously using meticulous techniques. It may be challenging for the coronary guidewire to enter the true lumen; however, the true lumen can be confirmed using intravascular ultrasound or optical coherence tomography before angioplasty or stenting.29,50 Due to the extensive nature of the dissections, long stents are often necessary despite the increased risk of restenosis.29,50 It is important to note that there is a risk of late stent thrombosis due to the chance of stent malposition after natural resorption of the intramural haematoma.50
Despite radial access being favourable in both STEMI and NSTEMI, femoral access is preferred in PCI for SCAD.50 This is because the radial approach has been associated with higher iatrogenic dissection rates.42,80 However, due to a lack of randomised data, there is no specific recommendation for P-SCAD (Table 1).
Secondary Prevention Post-PCI
ESC guidelines state that low-dose aspirin appears to be safe, but that clopidogrel should only be used when essential and for the shortest possible duration.7,13 Generally, little is known regarding P2Y12 inhibitors in pregnancy and, as such, use of P2Y12 inhibitors other than clopidogrel is not recommended.13 No complications have been reported so far in stented pregnant women treated with aspirin and clopidogrel, but breastfeeding is currently not recommended in women taking antiplatelet medications other than low-dose aspirin.13 There are currently no specific recommendations regarding the duration of antiplatelet therapy in pregnancy, but there is a suggestion in the ESC guidelines that the duration of dual antiplatelets for second- or third-generation drug-eluting stents can be shortened in pregnant women.13 An algorithm for the pharmaceutical management of AMI during pregnancy has been published.81
Timing and Mode of Delivery in Acute MI
ESC guidelines advise that the treatment of STEMI/NSTEMI should not be delayed for delivery.13 In an acute situation, the priority is to stabilise and treat the mother. The timing and mode of delivery (vaginal or elective caesarean section) must be decided based on maternal cardiac status and gestational age.13,20 Although neither mode of delivery is associated with a higher mortality, there is agreement in the literature that vaginal delivery is preferred as there are greater risks associated with anaesthesia and surgery.9,13,20 Based on the ESC guidelines, delivery should ideally be postponed for at least 2 weeks post-AMI as there is increased risk of maternal mortality during this period.13,20 It is critical that the multidisciplinary team, consisting of a cardiologist, obstetrician, anaesthetist and neonatologist, are involved in decision-making regarding treatment and delivery for a good outcome. Post-delivery, maternal monitoring should take place in a high-dependency or intensive care unit.20
Conclusion
Pregnancy increases the risk of AMI and its occurrence is likely to increase with the continuing trend of childbearing at older ages. SCAD is the most common cause of AMI presenting during pregnancy. A conservative approach is generally preferred in P-SCAD but PCI needs to be performed in specific cases for revascularisation. Special considerations should be taken into account before performing PCI due to the fragility of the arteries. According to the latest guidelines, PCI is the recommended strategy for revascularisation in pregnant women presenting with STEMI. Strategies to minimise the radiation dose should be employed and use of the latest-generation drug-eluting stents should be considered.
Many gaps in knowledge exist regarding AMI in pregnancy,
which are largely due to the rarity of the condition and low involvement of pregnant women in clinical research. However, large international population-based studies on P-SCAD, coronary embolism, coronary artery vasospasm and PCI in pregnancy are required to better understand the aetiology and optimal management of these conditions.