Since the introduction of percutaneous coronary intervention (PCI) for chronic total occlusion (CTO), conventional antegrade wiring (AW) techniques (‘true-to-true lumen’ approach) have been the traditional approach to tackle most cases.1,2 However, this has led to a premature plateau in success rates because this method is best suited for tackling simple-to-moderately complex occlusions. The introduction of subintimal tracking and re-entry (STAR) and the retrograde approach opened new avenues for operators to navigate beyond traditional intraplaque strategies. This allowed tackling occlusions previously deemed off limits or those that were usually unsuccessfully approached with conventional methods.3,4 This review focuses on antegrade and retrograde dissection/re-entry techniques for CTO recanalisation, providing a description on their technical aspects, as well as acute and longer-term outcomes.
Antegrade Dissection/Re-entry: Indications and Technical Aspects
There is a widespread consensus that AW is the preferred technique for cases with a short tapered proximal cap occlusion with a clear course, and a distal vessel of good quality.1,5,6 In fact, AW is the most frequently used and most frequently successful strategy across various practice patterns and approaches to PCI for CTO.7 However, when AW fails to reach the distal true lumen and acquires an extraplaque situation, antegrade dissection/re-entry (ADR) becomes a valuable bailout option. ADR can also be used as a first-line strategy in case of long (>20 mm) occlusions with an ambiguous or impenetrable proximal cap or, again, in case of an ambiguous or tortuous vessel course.1,3,5
As its name implies, ADR involves two components: dissection and re-entry.
Dissection
Dissection may occur inadvertently by advancing a wire in the extraplaque space or intentionally by knuckling a polymer-jacketed wire. The wire is advanced against resistance until its tip folds upon itself, acquiring the shape of a knuckle (or the tip is purposefully shaped by the operator into a ‘J’ shape). Wire selection may vary, typically involving low-tip load polymer-jacketed wires (such as the Fielder XT family) or specialised guidewires (such as the Gladius Mongo, which features an abrupt core tapering 8 mm from its tip: ‘knuckle point’). Conversely, higher-tip load polymer-jacketed guidewires (e.g. Abbott Vascular Pilot 200, Asahi Intecc Gladius and Teleflex Raider) tend to create larger knuckles. Although smaller knuckles (e.g. Gladius Mongo) are generally suitable for both dissection and re-entry, larger knuckles may be preferred during the dissection phase, in cases where avoiding side branches is crucial (e.g. acute marginal branches in the right coronary artery) or where a higher tip load is instrumental in overcoming fibrocalcified tissue. Although knuckling is considered generally safe, caution is advised, emphasising stepwise advancement and vigilant tracking in orthogonal projections to ensure proper knuckle propagation along the expected course of the main vessel, and avoiding deep advancement into smaller branches.
In case of an ambiguous or impenetrable proximal cap, the so-called ‘move-the-cap’ techniques can be used, the goal of which is to ‘go around’ a hostile cap. These techniques include balloon-assisted subintimal entry (BASE) and scratch-and-go.3 With BASE, balloon-induced intimal disruption is performed proximal to the proximal cap using a balloon sized 1:1 to the artery at high pressure. In case of significant fibrosis and/or calcification, speciality balloons can also be used, such as cutting/scoring balloons or even an intravascular lithotripsy balloon.8 With the scratch-and-go technique, the extraplaque space is accessed using a highly penetrative wire, proximal to the proximal cap, and the microcatheter is advanced. In both cases, the goal is to create intimal disruption and access the extraplaque space, to then knuckle a polymer-jacketed wire. In the case of a particularly resistant proximal cap, the balloon used for BASE can be reinflated as soon as the microcatheter is advanced into the extraplaque space so as to anchor the microcatheter and thus give more penetrative power to the knuckle (BASE power knuckle).9
Notably, BASE should be preferred to the scratch-and-go technique, whenever feasible, because the latter is technically more challenging and potentially dangerous, as the penetrative wire can inadvertently perforate the vessel. Finally, another approach to deal with a very resistant proximal cap is the gentle injection of a small amount of contrast via the microcatheter tip after nosing it inside the cap (Carlino technique), which will modify plaque compliance.3,10
Re-entry
Re-entry in the true lumen has been the holy grail of PCI for CTO ever since the introduction of ADR and is still the subject of intensive technical and technological research efforts. Re-entry can be achieved through various methods, which can be classified as wire-based (with or without intravascular imaging guidance) or device-based.
Wire-based Antegrade Dissection/Re-entry: Subintimal Tracking and Re-entry and Limited Antegrade Subintimal Tracking
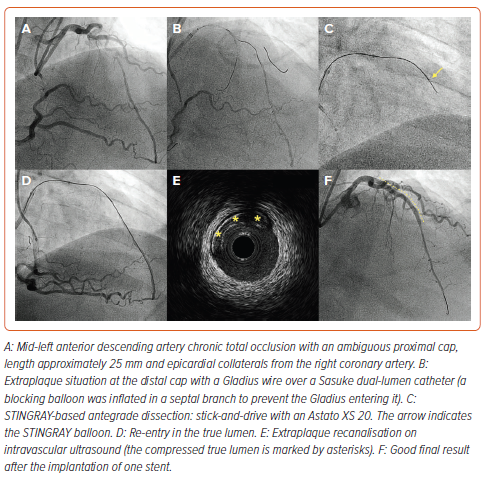
Historically, the first approach to re-entry consisted of simply continuing to advance the knuckle until it re-entered the true lumen. This technique was called STAR and was introduced by Colombo et al. in 2005.11 However, this technique was associated with very high rates of restenosis on short-term follow-up due to extensive dissection planes, loss of significant side branches and poor run-off.12,13 Subsequent modifications of the technique with novel wires (mini-STAR) or contrast injection to achieve hydraulic dissection of the vessel (contrast-guided STAR) were not able to significantly improve the outcomes.14 As such, STAR currently only serves as last bailout strategy in case of impending failure, with the goal of performing an investment procedure (Figure 1).15 Patients are typically brought back approximately 6–8 weeks later for a staged reattempt, allowing the dissection planes to heal before further intervention.16,17 This deferred stenting strategy has been associated with improved patency rates on follow-up.17,18
Another technique that is only rarely used nowadays in the context of wire-based ADR is limited antegrade subintimal tracking (LAST; 11.1% of ADR cases in PROGRESS-CTO).19 The primary goal of the LAST technique is to achieve a wire-based re-entry as close as possible to the distal cap by using a high-tip load and torqueable guidewire with a sharp bend at its tip.3 However, the LAST technique remains less predictable and reproducible, with lower technical success than device-based techniques.19–21
Intravascular Imaging-guided Re-entry
A modification of the LAST technique involves incorporating live intravascular ultrasound (IVUS) interrogation to guide re-entry. This is achieved via either a single 8-Fr guide catheter or a second guide catheter (Ping Pong or dual-guide technique).22–24 Very recently, a first-in-man case with a novel optical coherence tomography (OCT)-guided CTO re-entry device was reported, enabling real-time high-resolution visualisation with graphical augmentation and thus allowing precision steering and advancement of a guidewire.25
Device-based Antegrade Dissection/Re-entry: Antegrade Fenestration and Re-entry and Subintimal Antegrade Fenestration and Re-entry
The antegrade fenestration and re-entry (AFR) and subintimal antegrade fenestration and re-entry (SAFER) techniques aim to establish connections between the extraplaque space of a CTO segment and the distal true lumen through balloon-induced fenestrations. This is achieved by balloon dilation, and then guiding a wire through these openings. In AFR, a balloon sized 1:1 to the artery is advanced onto a first (extraplaque) guidewire and inflated across the distal cap. This will create transient fenestrations between the extraplaque space and the true lumen, and targeted re-entry into the true lumen can be achieved by engaging these fenestrations with a (second) polymer-jacketed guidewire.26 After its initial formulation, AFR showed moderate success rates of 65.9% in a multicentre registry.27 A more recent modification of AFR, called SAFER, involves the creation of larger and longer-lasting fenestrations between the false and true lumens through multiple inflations of the balloon along the entire occlusion, not just at the distal cap.28 Multicentre validation of SAFER is eagerly awaited.
Device-Based Re-entry: STINGRAY LP System and ReCross Microcatheter
A device-based approach aims to enhance the efficacy and reproducibility of re-entry procedures. The STINGRAY LP (Boston Scientific) was the first of such devices, featuring a flat balloon with two side exit ports positioned 180º apart, alongside a distal exit port. Upon gentle inflation, the STINGRAY self-aligns one of its ports with the true lumen. An angiographic projection parallel to the device is then sought. Subsequently, a highly penetrative tapered wire (e.g. Astato XS 20) is guided through the side port closer to the true lumen, facilitating successful re-entry (Figure 2).3,5 The same wire used to re-enter the lumen can then be advanced to the distal true lumen using a ‘stick-and-drive’ technique or, alternatively, it can be exchanged with a polymer-jacketed wire (e.g. Pilot 200) using a ‘stick-and-swap’ method (which is particularly useful in case of a diseased distal vessel). One challenge of this procedure is haematoma formation in the extraplaque space, which can be mitigated by burying a guide extension at the proximal cap before starting dissection manoeuvres. This issue can also be addressed by the subintimal transcatheter withdrawal (STRAW) technique, which involves attaching the over-the-wire STINGRAY port to a syringe and applying negative suction, which will evacuate the haematoma.
The ReCross microcatheter (IMDS) is uniquely designed as an oval-shaped microcatheter featuring two over-the-wire lumens and a total of three exit ports. The white hub connects to two of these ports: the distal one and the more proximal of two ports positioned 180º apart. The blue hub allows access to the second exit port (between those accessible via the white hub). The ReCross microcatheter can be used for device-based ADR, as well as for parallel wiring or as a conventional microcatheter.29
Retrograde Dissection Re-entry: Indications and Technical Aspects
Procedural algorithms favour the retrograde approach in the presence of interventional collaterals, an ambiguous proximal cap, a distal vessel of poor quality, a significant bifurcation at the distal cap or failure of antegrade techniques.3,30,31 Once collateral crossing is achieved, there are several options to complete CTO crossing. However, retrograde dissection/re-entry (RDR) is the most commonly used approach (63% in the PROGRESS-CTO registry).32
Although in one-third of cases operators may use retrograde wiring or a ‘true-to-true lumen’ approach (direct passage of the retrograde wire into the proximal true lumen without antegrade or retrograde balloon dilatation), this is often unsuccessful due to the high lesion complexity that dictated resorting to the retrograde approach in the first place.31,32 Therefore, in most cases, balloon dilatation is required to create a connection between the antegrade and retrograde systems. Two RDR techniques are available: controlled antegrade and retrograde subintimal tracking (CART) and reverse CART.
Controlled Antegrade and Retrograde Subintimal Tracking
CART was the first RDR technique to be introduced in 2005 by Katoh et al.33 With CART, an over-the-wire balloon was used to support retrograde guidewire crossing into the distal CTO vessel. Subsequently, retrograde balloon dilatation would facilitate antegrade wire crossing into the distal true lumen. CART was limited by the high crossing profile and the relative propensity of the over-the-wire balloons to kink. In fact, even in experienced hands, success rates were modest (40.8% in a single-centre study).34
The retrograde approach evolved over the following years and, in 2010, the Corsair microcatheter was introduced. With its ability to dilate retrograde channels, Corsair allowed a marked increase in the success rates of retrograde CTO recanalisation.4 This also allowed the introduction of reverse CART (described in the next section). Consequently, CART use dropped significantly, and reverse CART became the dominant retrograde CTO crossing technique.31,32,35
However, there are still situations where the original CART technique remains valuable:
- aorto-ostial occlusions with difficult guide engagement
- impenetrable proximal cap or any cases where the antegrade equipment cannot be advanced to overlap with the retrograde system
- failure of reverse CART; and
- when the retrograde equipment is not long enough to reach the antegrade guide catheter.36
In a contemporary single-centre registry, CART was used in a minority of very challenging (mean [±SD] J-CTO score 3.6±0.9) retrograde cases (7.5% of the overall retrograde cohort), most often in the context of impenetrable CTOs, as described above.36 The CART success rate was 73.3%, and overall technical/procedural success was observed in 82.2% of cases. Major adverse cardiovascular events (MACE) were observed in only 2.2% of cases (due to tamponade), with no fatalities, indicating the overall safety and efficacy of this technique in the hands of expert operators.36
Conventional Reverse Controlled Antegrade and Retrograde Subintimal Tracking
As discussed above, after the introduction of dedicated microcatheters, balloon dilatation of the occlusion could subsequently be performed in an antegrade manner, because retrograde wire manipulation and collateral channel and occlusion crossing were greatly facilitated by the microcatheter. The retrograde wire could then be advanced into the true lumen towards the antegrade guide catheter, the retrograde microcatheter was advanced into the antegrade guide catheter and externalisation of a >300-cm-long wire would follow. There are several other variations of reverse CART, which are discussed below.32
Intravascular Ultrasound-guided Reverse Controlled Antegrade and Retrograde Subintimal Tracking
Beyond determining the ideal balloon size to connect compartments effectively, IVUS proves helpful in evaluating antegrade and retrograde wire position within CTO segments for reverse CART troubleshooting.37 It can also provide live visualisation of the retrograde wire to assist in crossing and confirm wire location into the same space as the antegrade gear.38
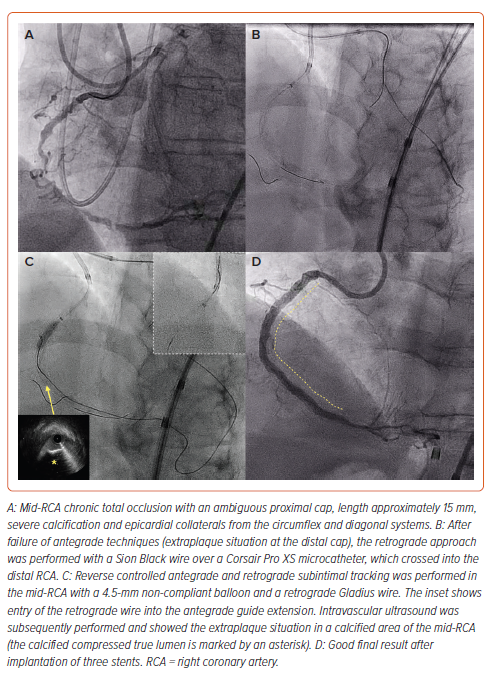
Guide Extension-facilitated Reverse Controlled Antegrade and Retrograde Subintimal Tracking
Advancing a guide catheter extension can provide an easier target for retrograde wire re-entry (Figure 3). This technique is routinely used (e.g. in one-fifth of retrograde cases in PROGRESS-CTO) and is particularly useful in the following scenarios:
- diffuse disease in the proximal vessel
- a retrograde microcatheter that cannot reach the antegrade guide catheter
- managing ostial left anterior descending artery or left circumflex CTOs to reduce the risk of extraplaque crossing into the left main coronary artery.32,39
Contemporary/Directed Reverse Controlled Antegrade and Retrograde Subintimal Tracking
Japanese operators advocate for a contemporary or directed approach to reverse CART, with the goal of achieving the least amount of vascular manipulation possible (under the belief of better long-term stent patency rates).40 In a directed approach, the goal is to achieve close proximity between the antegrade and retrograde systems, and for this purpose reliance is placed on highly torqueable wires (such as the Gaia family). A small balloon is advanced and inflated on the antegrade wire in the CTO segment and the retrograde wire aims at it.40 However, this technique may not be feasible in the presence of ambiguity of the vessel course or proximal cap, severe tortuosity or heavy calcification of the CTO vessel.
Extended Reverse Controlled Antegrade and Retrograde Subintimal Tracking
In this iteration, the operational base is relocated either proximal or distal to the CTO segment, and ballooning in those segments is performed to facilitate entry of the retrograde wire into the lumen.40 A potential drawback involves extending the dissection planes and the requirement for longer stents. Nonetheless, this approach can prove beneficial, particularly in the context of an impenetrable proximal or distal cap, especially in the absence of significant side branches.
Effect of Intraplaque versus Extraplaque Tracking on Vessel Patency Rates
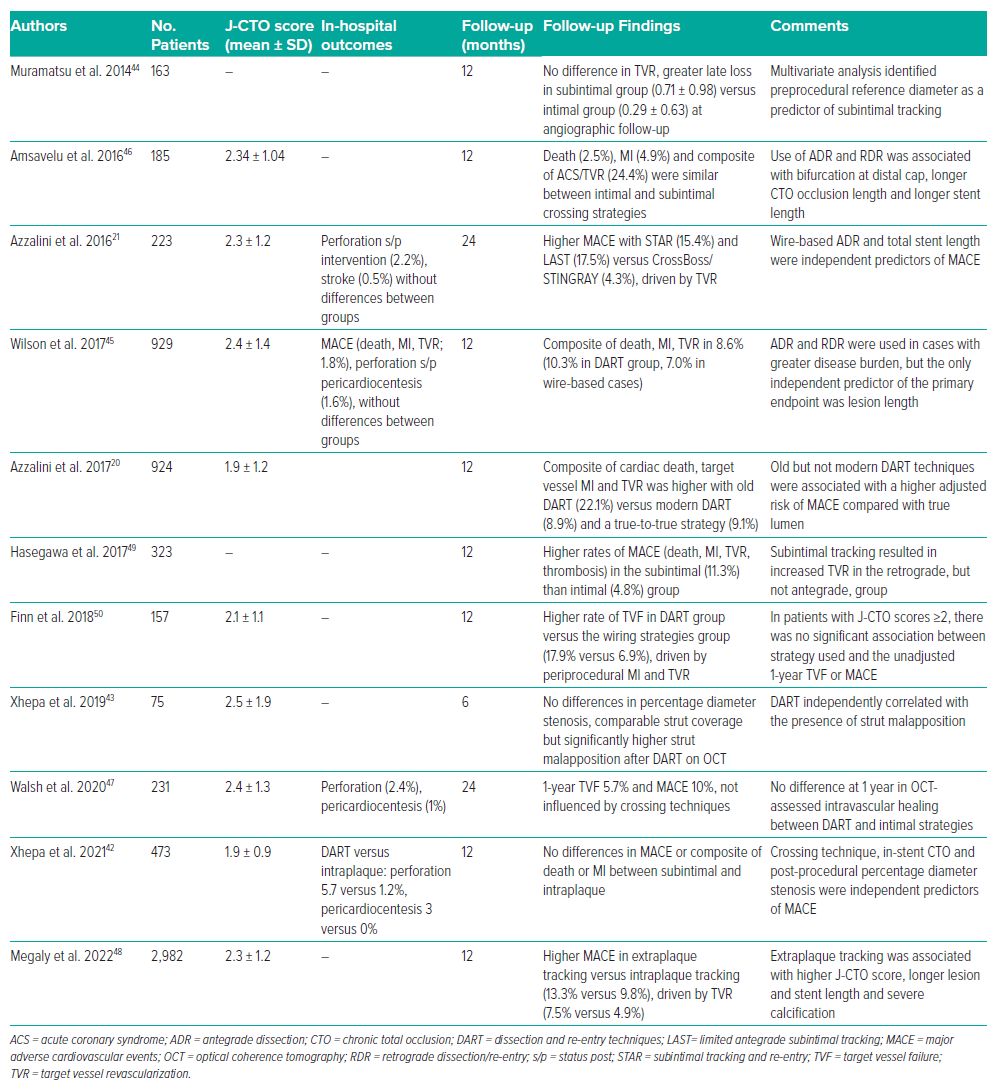
The subject of intense debate has been whether intraplaque versus extraplaque tracking during CTO recanalisation has a long-term effect on vessel patency rates. For many years, Japanese authors have, in fact, defended the importance of pursuing intraplaque tracking much as possible, due to the alleged impact on distal run-off and preservation of side branches. As such, their algorithm emphasises intentional wiring approaches.41 However, it is important to highlight that the intended use of dissection/re-entry techniques does not equate to extraplaque tracking, and even the intended use of a ‘true-to-true lumen’ approach does not necessarily lead to intraplaque tracking. In fact, IVUS studies have revealed that extraplaque tracking was observed in 27.9% of cases using an antegrade wiring approach, whereas intraplaque tracking occurred in 15.0% of cases using dissection/re-entry techniques.42 However, because intravascular imaging-based evaluation of wire tracking is not consistently performed in PCI for CTO literature, our discussion will sometimes have to take a simplified approach and compare dissection/re-entry techniques versus ‘true-to-true lumen’ wiring. A summary of the available literature on this topic is presented in Table 1.20,21,43–51
In a meta-analysis by Megaly et al., extraplaque tracking was more often used in complex CTO lesions and was associated with a higher risk of MACE (OR 1.50; 95% CI [1.10–2.06]; p=0.01), driven by a higher risk of target vessel revascularisation (OR 1.69; 95% CI [1.15–2.48]; p=0.01) at 1 year.49 However, there were no differences in terms of death or MI between extraplaque and intraplaque tracking.49
Similarly, other studies have reported a higher occurrence of target vessel revascularisation with extraplaque tracking, particularly in the RDR group, but not in the ADR group.48,50,51 Conversely, in the prospective CONSISTENT-CTO study, MACE rates at the 2-year follow-up were similar between dissection/re-entry and the ‘true-to-true lumen’ approaches.48
In an ADR approach, device-based re-entry, specifically using the CrossBoss/STINGRAY system, was associated with lower MACE rates compared with wire-based ADR techniques.20,21
Conclusion
Antegrade and retrograde dissection/re-entry techniques greatly expand and enhance the array of techniques available to CTO operators, offering solutions for challenging occlusions that were previously deemed too difficult to tackle or in which PCI was unsuccessfully attempted. Proficiency and ease in navigating the extraplaque space are imperative to ensuring a safe, effective and efficient approach to CTO revascularisation.