Dr Kapur opened with a case study of a 70-year-old active man who experienced chest pain during exercise. Electrocardiogram confirmed anterior ST-elevation MI (STEMI) with sum of ST elevation greater than 20 mm in leads V2–V5. The patient was admitted 1 hour from symptom onset. He consented to entry into the STEMI Door-to-Unload (DTU) clinical trial and was randomised to the control arm to receive standard of care for prompt revascularisation. Left ventricular (LV) assessment showed ejection fraction (EF) <30% and LV end-diastolic pressure 35 mmHg. Symptom-to-balloon time was 110 minutes and an optimal door-to-balloon time of <25 minutes was achieved. An ECG 3–5 days after percutaneous coronary intervention (PCI) showed restoration of LVEF to 50%; however, cardiac MRI (CMR) revealed that a massive anterior scar remained.
A population-based cohort study of 7,733 patients showed that a significant proportion of patients who experience their first MI after 65 years of age develops heart failure following revascularisation: 34% developed heart failure in hospital and 71% developed heart failure within 5 years.1 Dr Kapur used the case study and this reference to illustrate the disconnect between reperfusion and myocardial salvage: reperfusion alone may not achieve optimum myocardial salvage. These clinical observations led to the design of the STEMI-DTU trial, asking the central question of ‘Can we mitigate the onset of heart failure by further reducing the infarct size at the time of anterior STEMI and thereby preserving the myocardium for future years?’
Key prognostic factors to consider in STEMI patients include:
- infarct size and the extent of microvascular obstruction that occurs due to thrombotic debris;
- epithelium damage;
- vasoconstriction; and
- extrinsic compression of the microvasculature from oedema.
The STEMI-DTU pilot trial evaluated infarct size and the degree of microvascular obstruction after PCI in patients who had immediate reperfusion with Impella unloading versus delayed reperfusion after 30 minutes of Impella unloading. Dr Kapur noted that this trial tests a disruptive hypothesis that delayed reperfusion plus LV unloading limits myocardial ischemia and reduces myocardial damage. This novel strategy is based on a body of growing evidence that delaying reperfusion by 30 minutes while unloading achieves the lowest infarct size.3
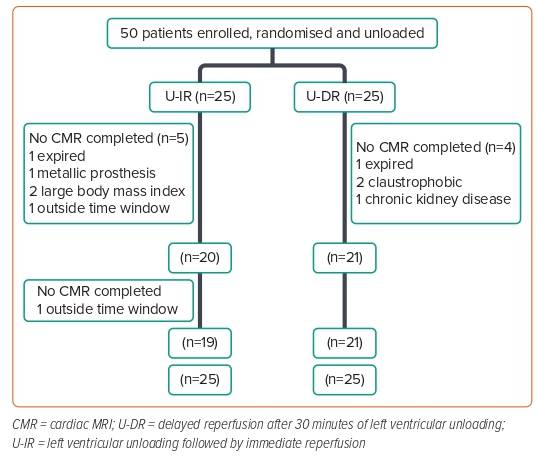
The pilot phase of the STEMI-DTU trial addressed the feasibility and safety of the strategy with Impella CP by assessing major adverse cardiac and cerebrovascular events at 30 days. The primary efficacy endpoint of infarct size/total LV mass at 30 days evaluated whether delaying reperfusion before LV unloading increased infarct size. Fifty patients were enrolled and randomised as per the study protocol shown in Figure 1.
Dr Kapur shared that the trial achieved 100% compliance for the protocolised 30-minutes unloading time before reperfusion in the LV unloading plus delayed reperfusion (U-DR) arm, which was a reassuring signal that the study approach is feasible. Dr Kapur highlighted a key learning from the pilot study, which is becoming more apparent in the ongoing pivotal study, namely that U-DR reduces ischaemic symptoms. The STEMI-DTU trialists coined the proverbial term ‘door-to-snore’ to describe their observations that upon Impella insertion, the patient’s crushing chest pain resolved to the extent that the patient was able to fall asleep while their left anterior descending artery (LAD) was still occluded. The results from the pilot trial showed that the U-DR approach uncoupled coronary occlusion from myocardial infarct size by creating a window of time, 30 minutes, that did not exist before. This window of time where LAD occlusion and ischemia are uncoupled allows for additional strategies to optimise myocardial and metabolic recovery to be implemented.
To understand the mechanism of U-DR, Dr Kapur and others conducted and published several preclinical mechanistic studies. A study of microcirculatory blood flow in an LAD infarct model showed that the U-DR approach enhanced coronary collateral flow before reperfusion and reduced the area of risk within the infarct zone.4 Using hypoxia-inducible factor (HIF)-1α as a biomarker of ischaemia, it was shown that LV unloading was associated with a decrease in HIF-1α expression prior to reperfusion that persisted over a period of 120 minutes.5 Metabolomics analysis of postinfarct myocardial tissue showed that transvalvular unloading triggers a global metabolic shift and reduces anaerobic glycolysis during coronary occlusion, which is a critical mechanism for ischemia–reperfusion injury (unpublished data). The current STEMI-DTU trial was protocolised with LV unloading for 30 minutes prior to reperfusion, but preclinical data from the Kapur laboratory and others suggest that a longer period of LV unloading prior to reperfusion may be feasible without increasing the ischemic burden.
Results from the STEMI-DTU pilot trial showed that across different sizes of anterior MI, the primary efficacy endpoint of infarct size/total LV mass was reduced in the U-DR arm compared with the unloading with immediate reperfusion (U-IR) arm. In addition, both the U-DR and U-IR arms had smaller average infarct size (22–27% and 17–19%, respectively) compared with the average infarct size of 36% in the CRISP-AMI trial populations.6 Microvascular occlusion showed the same trends: the U-DR arm achieved 1.3–1.6% microvascular obstruction after PCI, compared with 3.5–5.6% in the U-IR arm. These results demonstrate that 30 minutes of active LV unloading prior to reperfusion improves myocardial salvage compared with immediate reperfusion.
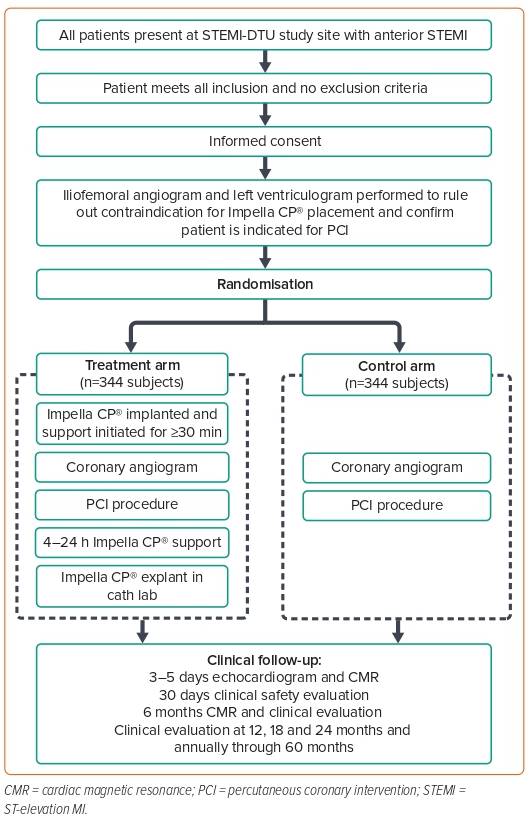
Dr Kapur concluded his talk by sharing the design of the STEMI-DTU pivotal trial, which is a prospective randomised multicentre trial. The STEMI-DTU pivotal trial is designed to compare mechanical LV unloading with the Impella CP device for 30 minutes prior to primary PCI to primary PCI alone without LV unloading, and to evaluate whether delayed reperfusion reduces infarct size and improves prognosis in patients with STEMI. All patients in the pivotal study will receive a femoral angiogram and a ventriculogram to ensure high-quality enrolment.
There was a significant study protocol non-adherence of 36% in the pilot trial due to the difficulties with study execution; for the pivotal trial, participating sites will have access to a community of clinicians available to provide support 24/7 to achieve greater study protocol adherence. The study protocol has been recently published (Figure 2),7 and recruitment is underway, with 250 subjects already enrolled.