Advances in transcatheter structural heart interventions and temporary mechanical circulatory support (MCS) have led to increased demand for alternative sites for large-bore vascular access.1 Percutaneous axillary artery (AA) access is an appealing alternative to standard femoral access when the patient has iliofemoral peripheral arterial disease (PAD), obesity or there is an expected device duration >24–48 hours. In the case of prolonged haemodynamic support, AA access for MCS device insertion allows for increased mobility while the device is in place, facilitating physical therapy and reducing morbidity associated with prolonged bed rest.2 Transaxillary access is now the most common alternative access site for transcatheter aortic valve replacement (TAVR) with balloon-expandable valves, with high rates of procedural success and similar outcomes whether a surgical or a percutaneous approach is taken.1
Historically, upper extremity large-bore arterial access required surgical cutdown to access the subclavian or axillary artery. A fully percutaneous approach has now been demonstrated and offers potential advantages of local anaesthesia and rapid access in an emergency, whereas surgical access typically requires a multidisciplinary approach potentially including anaesthesia, interventional cardiology and the cardiothoracic surgery team.1,3,4 A percutaneous approach targets the distal first or proximal second segment of the axillary artery, where it is compressible against the second rib. The selection of a surgical versus percutaneous approach should be determined by operator comfort the level of experience at the setting regarding management before and after the procedure.
Initial concerns regarding the safety of percutaneous AA access have not borne out in clinical practice.4–6 Cadaveric and clinical experiences demonstrate compressibility and when coupled with contemporary vascular closure devices, reliable haemostasis has been demonstrated without surgical cutdown after large-bore AA.7
This article outlines the basics of our approach to large-bore percutaneous subclavian and axillary vascular access, including patient selection and procedure planning, anatomic AA landmarks to guide access, access techniques, sheath removal and the management of complications.
Patient Selection and Pre-procedure Planning
Experience with AA access and several patient characteristics must be considered when selecting the site for large-bore access. Patients with significant iliofemoral PAD are the main candidates when an alternative access site is required and a centre is beginning to use the procedure. CT imaging in multiple TAVR populations has shown that the AA is often relatively spared from atherosclerosis in patients with severe iliofemoral PAD, but even a simple evaluation for pulses, blood pressure discrepancies and duplex ultrasound can be useful in the initial assessment for possible PAD in the upper extremity.8,9 AA access may also be advantageous in patients with obesity where the depth of the AA may be less challenging than the depth of the femoral artery (FA), depending on habitus. Additional clinical assessment for all patients should include anticoagulation status, the patient’s ability to cooperate during the procedure and other clinical characteristics that may affect the ability to access the axillary space, such as musculoskeletal deformities from previous injury, post-surgical changes or a permanent pacemaker/defibrillator. Factors that should be considered in laterality choice include the patient’s dominant hand, presence of a patent internal mammary artery graft and existing pacemaker or defibrillator. In these circumstances, contralateral AA access is preferred though ipsilateral access is not an absolute contraindication.
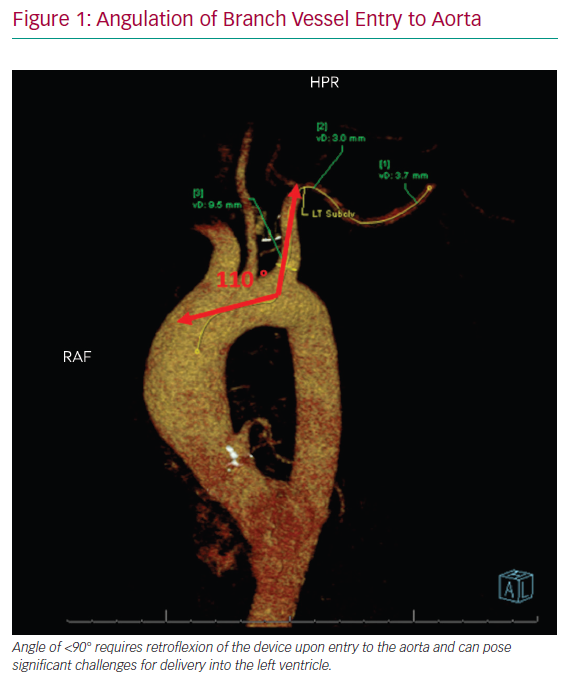
After careful clinical assessment, pre-procedural imaging is strongly advised. CT angiography (CTA), ideally with 3D reconstruction, is the preferred modality and should be performed in all patients. This allows for complete assessment of vessel calibre, degree of PAD, vessel tortuosity and angle of entry to the aorta. A vessel diameter of ≥6 mm is recommended to ensure adequate distal perfusion for indwelling devices, although ≥5.5 mm has also been suggested.8 Calcified vessels are more prone to dissection or distal embolisation and can lead to challenges deploying closure devices at the time of explant.10 For this reason, heavy calcification of the AA warrants additional consideration before large-bore access, aided by a detailed ultrasound assessment at the location of calcium within the arterial wall at the target site. The angle of take-off from the aorta and course of the innominate and left subclavian arteries and tortuosity should be considered when choosing laterality as these factors can affect the deliverability of the device (Figure 1). Angulation of the branch vessel to the aorta of <90° requires retroflexion of the device upon entry to the aorta and can pose significant challenges for delivery into the left ventricle. Keep in mind that CTA imaging is often performed with the patient’s arms above their head, which may create the appearance of a hinge point in the second segment of the AA that may be misread as a significant stenosis. Regardless, this location is often distal to the recommended access site.
Image guidance with point-of-care vascular ultrasound and/or direct peripheral angiography is also encouraged during the procedure and may be the only imaging performed in more urgent clinical scenarios. However, each of these modalities have limitations, which must be understood and a limited pre-procedural evaluation should be reserved for more experienced operators. While point-of-care ultrasound is strongly encouraged to assist with access, the assessment of vessel calibre compared with dedicated vascular ultrasound equipment is suboptimal. Additionally, point-of-care ultrasound does not allow evaluation of the more proximal vessel or aortic arch angles. Direct peripheral angiography provides adequate assessment of the aortic arch angles, though provides only a 2D assessment with limited evaluation of vessel calcification.
Anatomic Landmarks
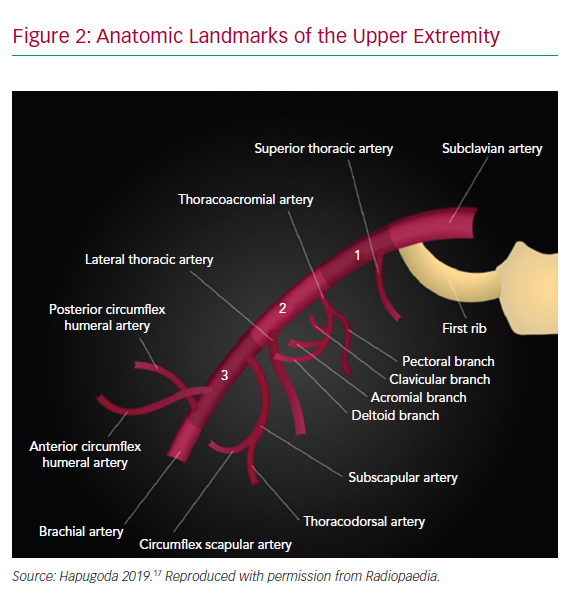
The AA originates as a continuum of the subclavian artery as it crosses beneath the clavicle lateral to the first rib and terminates in the brachial artery as it crosses the inferior border of the teres minor attachment to the humerus (Figure 2). Branches off the AA include the highest thoracic, lateral thoracic, anterior circumflex humeral, posterior circumflex humeral, thoracoacromial and subscapularis arteries which often provide collateral flow distal to the access point for indwelling devices.2 Access is typically obtained in the first segment of the AA to allow for vascular compression against the second rib and to avoid many of the branch vessels, although the skin puncture is performed more distally to allow for a shallow angle of entry.11 Injury to the brachial plexus is also more easily avoided in this region as the nerve bundle travels cephalad to the AA and can be identified on ultrasound.12
Access Technique
There are various iterations of AA access technique.3,6,9,11,13 Here, we review the key steps for successful percutaneous AA access. As discussed above, pre-procedural imaging with CTA or a combination of ultrasound and fluoroscopically guided access are critical aspects for procedural success.
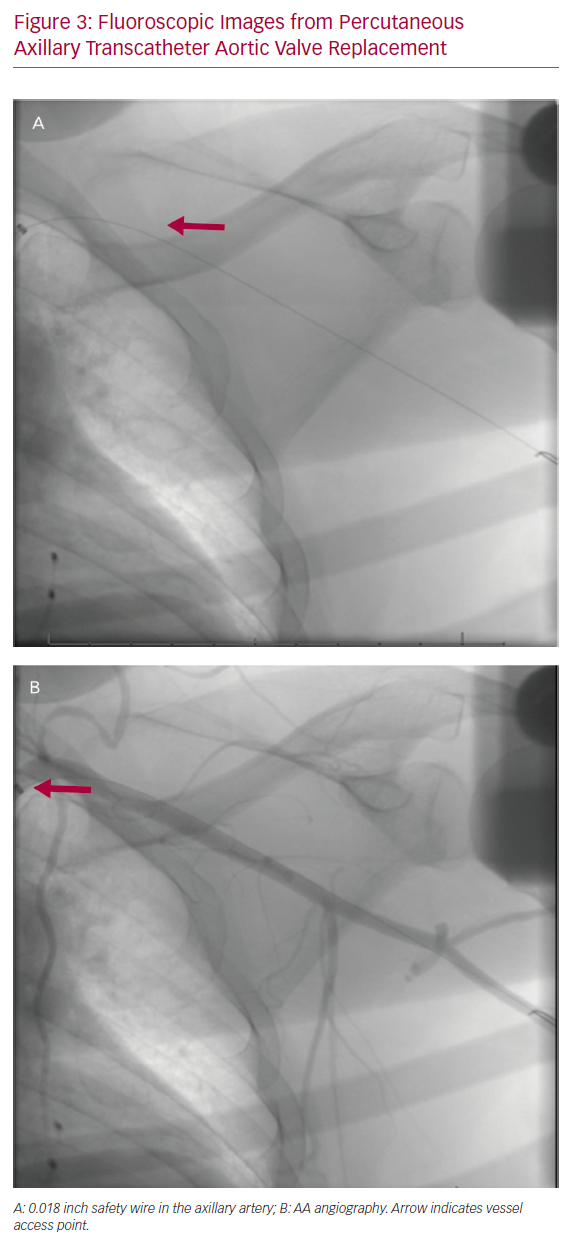
Prior to AA puncture, femoral or ipsilateral radial arterial access is obtained using a standard 5 Fr or 6 Fr catheter in order to perform AA angiography. The FA is generally preferred in our practice, as antegrade angiography will provide superior visualisation of the arterial structure. The FA also allows the ability to upsize the sheath, offering a broader range of bailout strategies when necessary. Following FA or radial artery access, a spring-coil tipped 0.018 inch wire is advanced across the AA to the brachial artery in the case of FA access or into the ascending aorta in the case of radial access. This serves as a fluoroscopic marker of the AA and is used to map the arterial course on the skin using the lung fields and the lateral border of the second rib as fluoroscopic landmarks, noting that the AA typically runs cephalad to the deltopectoral groove (Figure 3). The 0.018 inch wire is also used as a safety wire, allowing for prompt advancement of a balloon should vessel tamponade be necessary.
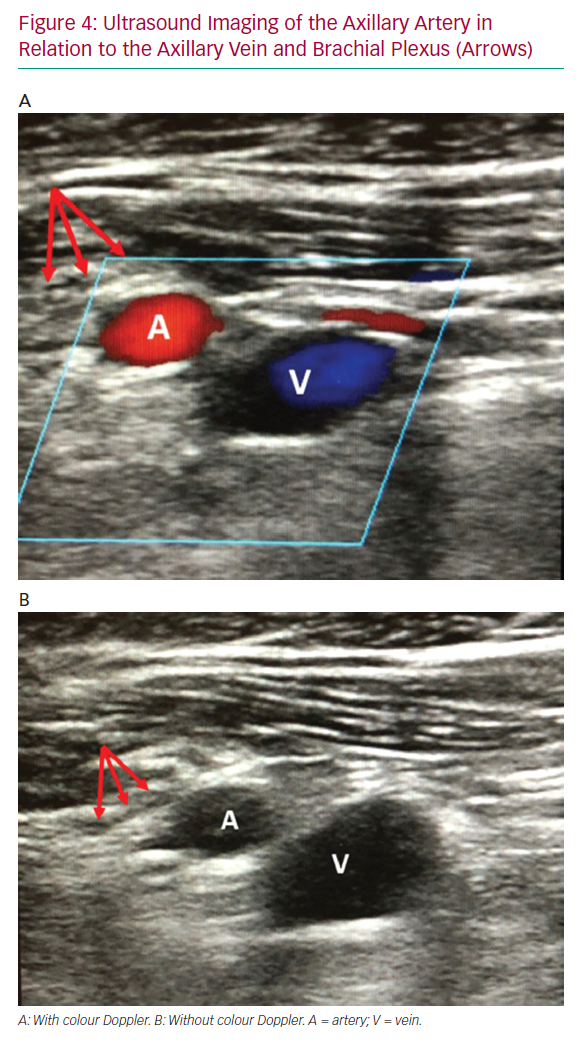
Following peripheral angiography and fluoroscopic AA mapping, the proximal segment of the AA should be identified on ultrasound with respect to the subclavian vein and brachial plexus (Figure 4). This is the preferred segment for percutaneous access due to compressibility and there being fewer adjacent structures. If the vein appears on top of the artery, tilting the ultrasound probe laterally away from the sternum and clavicle will demonstrate that the AA runs cephalad to the vein more laterally at the optimal puncture site. The thoracoacromial branch of the AA may also be seen and therefore avoided when using ultrasound to facilitate access. Once the access segment is identified, use ultrasound-guided micropuncture technique at a shallow angle of entry (about 30°) to access the AA near the lateral border of the second rib on fluoroscopy. Based on our experience, the skin entry point is usually just medial to and at the inferior aspect of the deltopectoral groove. A shallow angle of entry is of the utmost importance to allow large-bore device delivery and avoid kinking of the sheath as it advances under the clavicle. This also minimises protuberance of an indwelling system and tension on the arteriotomy site and leads to less oozing around the device with longer-term use.
Once acceptable access is obtained, the micropuncture should be upsized to a 6 or 8 Fr arteriotomy over a standard 0.035 inch J-tipped wire. This will facilitate placement of one or two 6 Fr Perclose ProGlide devices (Abbott Vascular), depending on anticipated arteriotomy size, which are used to ‘pre-close’ the arteriotomy and facilitate haemostasis at the time of explant. If two Perclose devices are required, we recommend the sutures be deployed at minimal angulation – at 11.30 and 12.30 positions rather than the classic 10 o’clock and 2 o’clock – as a milder degree of angulation is better tolerated in the AA. Once the vessel has been pre-closed, the 6 or 8 Fr sheath can be inserted and a standard diagnostic catheter and a stiff 0.035 inch wire are used to traverse the aorta and aortic valve. Following acceptable positioning of the stiff 0.035 inch wire placement in the desired location, the large-bore sheath can be delivered in standard fashion with manual pressure or internal balloon tamponade used for haemostasis between dilators where required.
Removal and Closure
When AA access is used for procedural indications such as transcatheter structural heart interventions or haemodynamic support during protected percutaneous coronary interventions (PCIs), the sheath can be removed at the end of the case and the Perclose sutures tightened in the usual fashion. When closure is delayed, as in cases of prolonged haemodynamic support, the patient is brought back to the cardiac catheterisation lab and 6 Fr FA access is obtained to facilitate angiography and bailout strategies at the time of explant if possible. Baseline angiography of the subclavian artery is performed before device removal to evaluate vessel patency, distal flow to the ipsilateral arm and thrombus at the insertion site.9
Depending on the device and sheath in the place from the AA, re-establishing wire access through the arteriotomy may require specific tips beyond the scope of this article.14 Nevertheless, re-establishing wire access for any closure device beyond manual pressure should be considered optimal care. Internal balloon tamponade for a dry field closure is also best practice, particularly when an operator is unfamiliar with the procedure. This can be performed with a peripheral balloon sized 1:1 with the distal subclavian (typically 6–8 mm) and inflated to no more than nominal pressures.
When feasible, our favoured approach is to ‘pre-close’ the arteriotomy using the Perclose ProGlide device at the time of device implantation. Using this technique, sutures can be tightened at the time of device removal to facilitate vessel closure. In the absence of a pre-closure, vascular closure devices can be deployed at the time of device removal although it is often more difficult. Suture-mediated closure remains the most common form of haemostasis, however, vascular plugs, or a combination of suture and vascular-plug-mediated closure have been described and can be used at the discretion of the operator.15 The primary advantage of a suture-mediated vascular closure device is maintenance of wire position in the vessel to allow for additional haemostasis options if necessary as well as not necessitating a foot plate within the vessel. Once adequate haemostasis is achieved, final angiography of the subclavian and axillary artery is performed using a standard catheter through the FA sheath to ensure vessel patency, adequate haemostasis and absence of obstructive thrombus.
Management of Complications
As with any large-bore arterial device, bleeding complications can occur. Mild bleeding around the arteriotomy site is not uncommon, especially in the setting of prolonged device implantation. Maintaining the initial insertion angle and a mild degree of cranial-medial tension on the sheath can limit minor bleeding. If needed, the Perclose sutures placed during the initial insertion can be tightened (‘cinched’ around the indwelling device) to achieve haemostasis. More clinically significant bleeding complications include the development of a hematoma, which invariably occurs along the pectoralis or lateral chest wall. The AA, at the point of optimal entry is not intrathoracic and occult thoracic bleeding is not a feature of arteriotomy bleeding from the AA. Branchial plexus complaints are rare and appear more likely to be related to external compression from the development of haematoma rather than the initial access, highlighting the importance of effective haemostasis. Early multicentre data following axillary Impella (Abiomed) implantation reported bleeding and haematoma in 6% of patients which is consistent with findings from other large-bore access registries.7
Bleeding from inadequate haemostasis at the time of explant is often visualised by invasive angiography. Balloon tamponade performed via FA or ipsilateral radial access or manual pressure are typically sufficient to achieve adequate haemostasis should the closure devices fail, particularly in conjunction with reversal of any anticoagulation. Covered stents can be deployed but are rarely needed and are associated with unclear long-term durability. As such, covered stents are reserved for severe refractory bleeding.
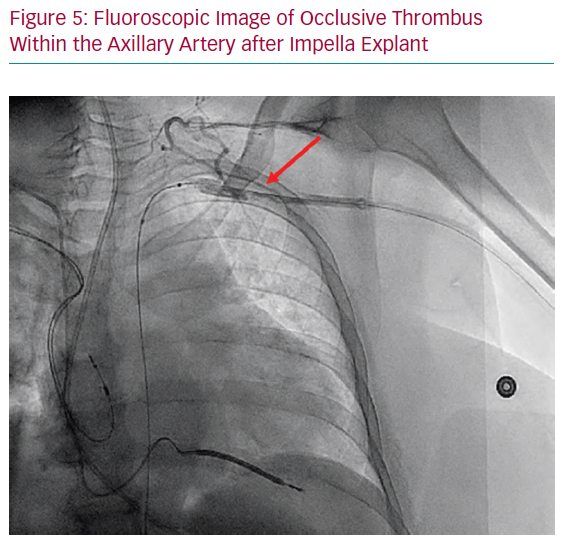
Laminar thrombus formation around the indwelling device is a more frequent complication in the case of prolonged indwelling devices, though distal flow to the extremity is often preserved via robust collateralisation, and thus this is primarily a radiographic rather than a clinically apparent phenomenon.2 It is our practice that if occlusive thrombus is observed (Figure 5), balloon angioplasty and, occasionally, thrombectomy may be required if the thrombus is displaced distally and becomes occlusive. In the case of non-occlusive thrombus noted on angiography with in-line perfusion to the extremity, the patient can be treated with 48–72 hours of unfractionated heparin with regular neurovascular examinations.
It is worth emphasising that two recent studies demonstrated higher stroke rates in patients undergoing TAVR via an AA approach compared with the high and intermediate risk cohorts in the Placement of AoRTic TraNscathetER Valves (PARTNER) II trial.1,16 It remains unclear if this is due to patient-related factors, including peripheral vascular disease requiring alternative access, or crossing the head and neck vessels, and interestingly it was not associated with mortality.1 Further investigation into the mechanism and risk of stroke with surgical and percutaneous AA approaches, including indwelling devices, is warranted.
Conclusion
Percutaneous AA access carries unique advantages of improving mobility in patients with indwelling mechanical support devices and avoiding suboptimal or hostile iliofemoral arteries in the case of PAD. The AA may be limited by size in certain patients and pre-procedural imaging is required in some form to assess for adequate size, atherosclerotic burden, and inhospitable angulation with the aortic arch. The rapid growth and evolution of transcatheter structural heart interventions and temporary MCS devices requiring large-bore arterial access continues to drive a need for ‘alternative’ access sites. AA access as described here is a teachable technique and it is a powerful skill to have while striving to provide the best care for patients. While this review provides a basic outline of the necessary steps for success, the importance of operator comfort and experience cannot be overstated. Before initiating a fully percutaneous AA access programme, seeking advice from someone with more experience or attending a dedicated hands-on programme is strongly encouraged to ensure good outcomes with early practice.