COVID-19, an acute respiratory syndrome resulting from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, was declared a public health emergency of international concern by the WHO in January 2020 and declared a pandemic by March 2020 due to its rapid global spread. While the primary impact of SARS-CoV-2 is on the respiratory system, potentially progressing to acute respiratory distress syndrome, recent research highlights significant extrapulmonary manifestations of COVID-19.1,2 A notable number of COVID-19 patients develop a hypercoagulable state, which increases the risk of venous and arterial thrombosis.3 This condition can lead to acute mesenteric ischaemia (AMI), a condition associated with significant morbidity and mortality. While the incidence of AMI in the general population ranges from 0.09% to 0.2%, this rate increases to between 1.9% and 3.8% in COVID-19 patients.4,5
Traditionally, open surgery was the standard treatment in AMI patients with clear evidence of peritonitis or bowel infarction or necrosis on CT, allowing for direct assessment of bowel viability and the removal of necrotic segments.6 However, advancements in diagnostic capabilities and imaging techniques have facilitated more rapid diagnosis of AMI, prompting a shift towards endovascular interventions as the frontline therapeutic approach for patients exhibiting a gradual onset of symptoms and the absence of peritonitis.7 Moreover, the benefits of endovascular treatments, including shorter hospital stay and reduced procedure time, could help minimise the spread of COVID-19.8
In this report, we present two cases of COVID-19 patients who experienced superior mesenteric artery (SMA) thrombosis and were effectively treated through endovascular intervention.
Case Presentation
Case 1
A 52-year-old man presented to the emergency department (ED) with persistent, dull epigastric abdominal pain of 2 hours duration, which had no identifiable precipitating factors. He had been diagnosed with mild COVID-19 infection 5 days prior to admission and was managed on an outpatient basis without specific therapeutic interventions. The patient had a 20-year history of smoking but no prior history of cardiovascular disease.
Upon admission, the patient was alert and without dyspnoea. His pulse rate was 86 BPM, maintaining regular rhythm. Blood pressure was 110/60 mmHg and oxygen saturation was 98% in ambient air. Additionally, the patient’s body temperature was 37.0°C. Clinical assessment revealed epigastric tenderness without guarding, with stable vital signs and a BMI of 25.8 kg/m2. Initial laboratory findings indicated normal white blood cell counts, hypercholesterolaemia, markedly elevated D-dimer levels, and a positive result for SARS-CoV-2 on reverse transcription-polymerase chain reaction (RT-PCR) testing. Other laboratory parameters were within normal limits. Abdominal ultrasound revealed hepatic steatosis without additional abnormalities.
The patient initially presented with gastrointestinal symptoms and was diagnosed with gastritis. Treatment commenced with a proton pump inhibitor and antacid. However, the abdominal pain persisted after 2 hours of treatment, prompting consideration of alternative diagnoses, including acute mesenteric ischaemia, perforated bowel, or bowel obstruction. Other conditions, such as abdominal aortic aneurysm, cholecystitis and pancreatitis were ruled out based on normal ultrasound and laboratory findings. A contrast-enhanced abdominal CT was performed to investigate the underlying cause. The CT scan revealed severe atherosclerotic stenosis in the coeliac arteries (approximately 80% in the initial segment) and occlusion of the superior mesenteric artery, with no evidence of bowel necrosis.
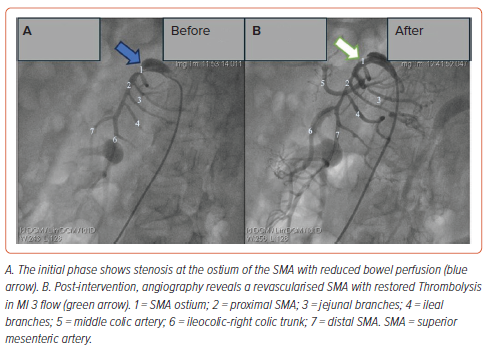
The patient was promptly referred to our department for digital subtraction angiography. A 6 Fr sheath (Prelude, Merit Medical) was inserted into the right common femoral artery, followed by the administration of 5,000 IU of heparin. Angiography of the mesenteric artery system using a selective catheter (Soft-Vu, AngioDynamics) identified 95% stenosis at the ostium of the SMA. A 0.014" guidewire (Regalia) was placed through the stenotic segment, and balloon angioplasty was conducted using a Sapphire balloon (3.75 mm × 10 mm) at 14 atm pressure at the stenosis site under fluoroscopic guidance. Subsequently, a 4.0 mm × 18 mm balloon-expandable bare metal stent (EucaLIMUS, Eucatech) was deployed, followed by additional balloon dilation extending from the SMA ostium to the proximal end of the stent. The final angiogram demonstrated successful stent expansion and restoration of blood flow to a thrombolysis in MI (TIMI) 3 level (Figure 1). The procedure was completed within 1 hour without any observed complications.
Following the procedure, the patient was transferred to the cardiovascular care unit and initiated on dual antiplatelet therapy (aspirin and clopidogrel), rivaroxaban and atorvastatin. He was discharged 3 days later without encountering any in-hospital complications and was scheduled for monthly follow-up appointments as an outpatient.
Case 2
A 58-year-old man presented to the ED with acute epigastric pain that commenced 3 hours after dinner. This visit occurred 1 week after his discharge for moderate COVID-19. His past medical history included hypertension, dyslipidaemia and type 2 diabetes, managed with losartan, atorvastatin and metformin. The patient had no history of smoking.
During examination, the patient presented with mild epigastric distension and tenderness without abdominal guarding. The patient denied experiencing other symptoms, such as cough, fever or dyspnoea. Vital signs were stable, with a regular pulse rate of 98 BPM with rhythm, oxygen saturation at 96% in room air, and blood pressure measuring 140/80 mmHg. His BMI was 20 kg/m2. Laboratory investigations revealed elevated triglyceride levels and HbA1c, along with a mild increase in D-dimer. COVID-19 testing by RT-PCR was negative. Other haematologic parameters were within normal ranges. Abdominal ultrasound findings were unremarkable, while chest X-ray demonstrated signs of pulmonary fibrosis secondary to previous COVID-19 infection.
The initial diagnosis for this patient was gastritis. Following laboratory tests and ultrasonography, other potential differential diagnoses, such as MI, cholecystitis, pancreatitis, and bowel obstruction were ruled out. However, given the persistence of the patient’s pain over several hours accompanied by symptoms of nausea and vomiting, an abdominal CT scan with contrast was subsequently conducted. The result showed widespread calcification in the abdominal aorta and iliac artery, significant stenosis of the coeliac artery, and the superior mesenteric artery ostium without sign of small intestine necrosis.
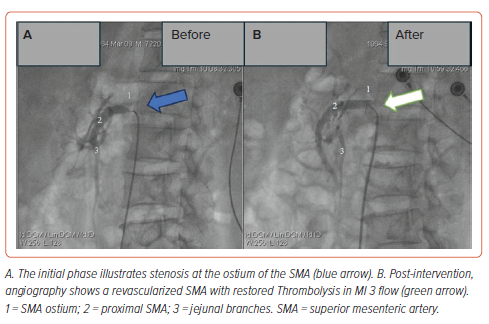
Following the diagnosis of AMI, he was promptly transferred to the interventional suite. A 6 Fr sheath (Prelude, Merit Medical) was inserted into the right common femoral artery, and 4,000 IU of heparin was administered. Selective angiography of the mesenteric arteries using a Soft-Vu catheter (AngioDynamics) revealed a 95% stenosis at the ostium of the SMA. Subsequently, a 0.014" guidewire (Regalia) was advanced across the narrowed segment, followed by balloon angioplasty using a JADE balloon (5.0 mm × 20 mm) at 12 atm pressure and deployment of an expandable bare metal stent measuring 7.0 × 25 mm (Dynamic, BIOTRONIK) at the stenosis site. Post-procedure angiography confirmed successful stent expansion and restoration of TIMI 3 flow (Figure 2). The procedure duration was 1.5 hours without any complications.
The patient demonstrated clinical improvement 3 days post-procedure and was discharged from the hospital accordingly. The treatment regimen included dual antiplatelet therapy (aspirin and clopidogrel), rivaroxaban, atorvastatin, losartan, and metformin. Follow-up appointments were scheduled monthly in an outpatient setting.
In both cases, the anticoagulant medication (rivaroxaban) was stopped after 1 month. At a 6-month follow-up after the procedure, both patients showed no signs of symptoms and continued with other medications.
Discussion
In COVID-19 patients, there is an established risk for AMI. Nonetheless, the underlying pathophysiology of the association between COVID-19 and AMI remains poorly understood. Initial findings indicate that thrombosis in the small mesenteric vessels, rather than embolic events, may be more prevalent in COVID-19 patients.9 Hypercoagulability demonstrates a strong correlation with hyperinflammation. During SARS-CoV-2 infection, patients often experience a systemic, intensified, and prolonged inflammatory response, commonly referred to as a ‘cytokine storm’. This overwhelming inflammatory response can disrupt the body’s natural anticoagulant mechanisms, precipitating abnormal development of thrombus, impaired fibrinolysis, endothelial damage, and excessive platelet activation.10
Other pathological mechanisms include the disruption or dysregulation of endothelial cells, which may enhance vascular thrombosis through the release of von Willebrand factor from Weibel-Palade bodies.11 The targeting of endothelial cells by SARS-CoV-2 through the expression of angiotensin-converting enzyme 2 (ACE2) and the binding of SARS-CoV-2 to ACE2 result in a decreased conversion of angiotensin II into angiotensin 1–7.12,13 This process leads to an increase in the level of vasoconstrictive agents, contributing to a hypercoagulable state. Moreover, additional factors have been recognised to exacerbate the hypercoagulable state of SARS-CoV-2 infection, encompassing male sex, hypertension, cardiovascular comorbidities, obesity and prolonged periods of immobility.14 Thromboembolic events associated with COVID-19 pose significant risks for mortality and morbidity. Many studies have shown that COVID-19 patients with intestinal ischaemia had a mortality rate as high as 60%.15 Rapid identification and prompt management of this condition are essential to decrease morbidity and mortality in COVID-19 patients. Normally, the intestines can tolerate a 75% reduction in blood flow for up to 12 hours due to the extensive network of mesenteric collateral vessels.16 Prolonged ischaemia beyond this threshold can progress to necrosis, perforation, peritonitis and ultimately, fatality. Therefore, the therapeutic approach for AMI focuses on revascularisation to halt the progression of ischaemia towards intestinal necrosis.
The management of AMI in COVID-19 patients poses significant challenges, given the absence of established guidelines or consensus statements. Individualised strategies should be adopted based on symptom severity and patient-specific factors, such as the aetiology of AMI, presence of peritonitis, and haemodynamic stability. When feasible, endovascular therapy is favoured as an initial approach over surgical interventions like laparotomy, offering superior outcomes in terms of both in-hospital mortality and overall morbidity.17 Endovascular treatment options encompass percutaneous aspiration, endovascular thrombolysis, and percutaneous transluminal angioplasty with stenting.18 In cases where patients presented with signs of peritonitis, necrosis or intestinal perforation, urgent laparotomy is recommended.16
In this report, we present two cases of COVID-19 patients who developed SMA thrombosis. Both patients had predisposing factors for thrombotic events. The first patient had long-term smoking habits and obesity, while the second had pre-existing hypertension and diabetes. At the ED, the diagnosis of AMI was promptly prioritised due to the presentation of epigastric pain and nausea that persisted despite proton pump inhibitors and antacid therapy. With no signs of intestinal necrosis from clinical and imaging assessments, the decision was taken to proceed with endovascular intervention. Following the procedure, both patients demonstrated favourable post-interventional outcomes. Anticoagulant therapy was maintained for 1 month, followed by dual antiplatelet therapy. While certain studies advocate for extended thromboprophylaxis following discharge post-COVID-19, there is no consensus on this aspect.19 Considering the stable condition of these patients, the decision was made to discontinue anticoagulant medications after 1 month.
Multiple cases of acute superior mesenteric ischaemia (SMA) have been reported in COVID-19 patients. Most of these patients underwent laparotomy, with a common characteristic being the extended postoperative course. In 2022, Sukegawa et al. reported a case where SMA occlusion occurred 8 days following severe COVID-19 pneumonia and a right renal infarction. The medical intervention included a necrotic bowel resection, the creation of a jejunostomy, and a transverse colon mucous fistula. The patient was discharged on the 45th day and resumed normal social activities 6 months post-surgery.20 In another instance, Aryal et al. reported on a healthy male who developed SMA 10 days post-COVID-19 infection, confirmed by CT scans showing bowel necrosis. This patient required an emergency exploratory laparotomy and was released on the 35th day post-operation.21 These cases illustrate that laparotomy is often necessary for COVID-19 patients with SMA and bowel necrosis. Although our observations indicate that prompt diagnosis and endovascular treatments can improve outcomes, these conclusions are based on a limited number of cases and should not be universally applied. This emphasises the necessity for more extensive and prolonged studies to corroborate these preliminary findings.
Study Limitations
Given the overlapping atherosclerosis risk factors observed in our patients, the connection between AMI and SARS-CoV-2 infection may not be clearly defined in our studies. The presence of these common risk factors could pose challenges in distinguishing the specific pathophysiological processes underlying SMA thrombosis in these patients. Moreover, due to the lack of a widely accepted treatment protocol for this condition, our approach only represents a practice based on experience and should be evaluated in further studies.
Conclusion
Acute mesenteric ischaemia poses a substantial, potentially life-threatening challenge, especially among individuals with COVID-19. Timely identification of this complication, followed by prompt intervention, is imperative for reducing mortality rates. For patients lacking evidence of intestinal necrosis, endovascular interventional techniques are emerging as safe and effective alternatives to conventional open surgical approaches.