Fractional flow reserve (FFR) is an index used to describe the physiological significance of a coronary stenosis. It measures the pressure drop under conditions of maximal hyperaemia between the aorta and a selected vessel location distal to an angiographic lesion.1 Several FFR studies have identified patient groups in whom coronary stenting is associated with improved outcomes when compared with medical therapy (and vice versa). FFR-guided treatment prevents unnecessary stenting and therefore coronary physiology has been associated with improved outcomes at reduced cost, a rare combination in a new technology.2–7 Coronary physiological assessment is now an integral part of the modern catheterisation laboratory, with a level 1A recommendation by the European Society of Cardiology (ESC) for use in stable patients in whom evidence of ischaemia is not already available.8 Routine use of FFR, however, requires some understanding of the strengths and weaknesses of the technology, to best apply the results obtained from the individual patient on the catheter laboratory table.
Ischaemic Heart Disease and Coronary Revascularisation
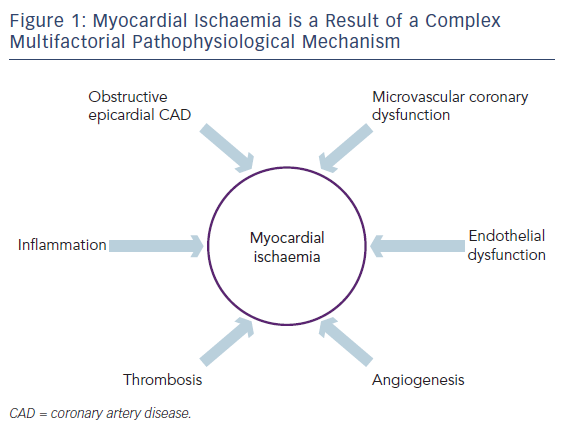
Angina is the clinical manifestation of myocardial ischaemia in patients with stable coronary artery disease (CAD). It is caused by transient imbalance between blood supply and metabolic demand.9 The myocardial ischemia that causes angina is, however, not simply a question of a ‘blocked pipe’; rather, ischaemia is a result of complex pathophysiological mechanisms that include obstructive epicardial CAD, inflammation, microvascular coronary dysfunction, endothelial dysfunction, thrombosis and angiogenesis (see Figure 1).10
Obstructive epicardial CAD is only one of the factors that contributes to myocardial ischaemia and yet, in the treatment of ischaemic heart disease (IHD), great (some would say overwhelming) emphasis is given to identifying and eliminating obstructive epicardial CAD over other potential causes of ischaemia, some of which may have key therapeutic roles in treating patients with IHD. The reason for this emphasis is obvious – obstructive epicardial CAD is easily identified, easily understood and relatively easily treated by angiography and stenting or bypass grafting. Doing so, however, does not always relieve ischaemia.
Concept and Validation of Fractional Flow Reserve
When FFR is used to identify a potentially ischaemia-causing lesion, the aim is to identify deficient blood flow distal to a coronary lesion. However, unlike coronary pressure, measurement of coronary blood flow in the catheter laboratory is not technically straightforward. Given the theoretical linear correlation between pressure and flow under conditions of minimal, constant coronary microvascular resistance, the pressure traces that we acquire with a standard coronary pressure wire are used to estimate blood flow; pressure wires do not directly measure blood flow. This theoretical linear relationship between blood pressure and flow is achieved by inducing maximum hyperaemia in the distal bed through pharmacological means, which lowers microvascular resistance, rendering it of minimal significance within the equation governing the relationship between blood pressure and flow within a closed fluid-filled system.11
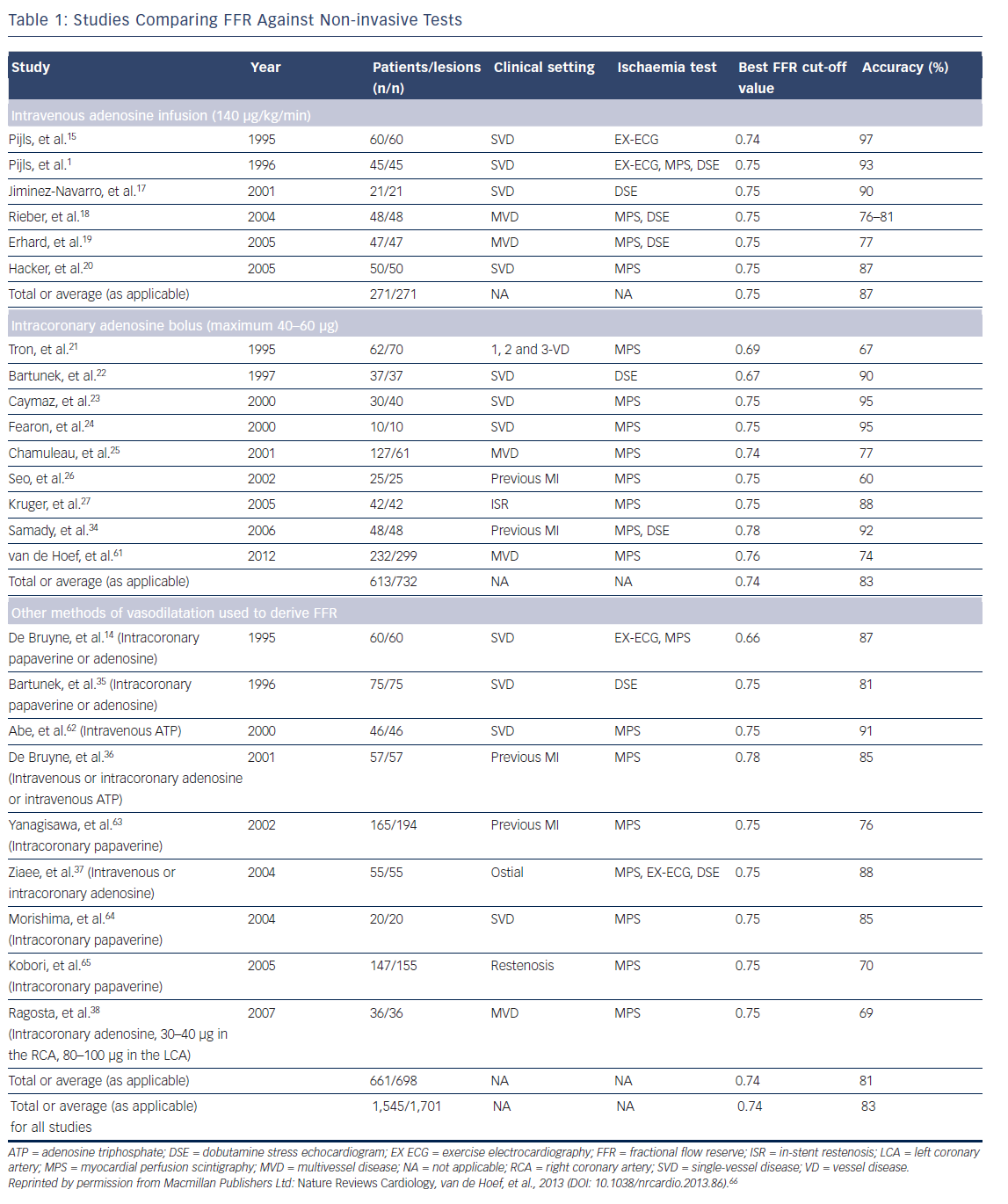
Since the initial development of the tool, FFR has been comprehensively validated as an invasive physiological index of ischaemia against other non-invasive modalities, including positron emission tomography, dobutamine stress echocardiogram and exercise stress testing (see Table 1).12–16 Furthermore, FFR values have been demonstrated to improve and non-invasive evidence of ischaemia resolve after revascularisation.
The initial equation for FFR measurement (FFR=mean distal coronary pressure minus mean central venous pressure divided by mean aortic pressure minus central venous pressure [Pd–Pv/Pa–Pv] measured during stable, steady-state hyperaemia) incorporated central venous pressure values, as right atrial pressure impacted on the transmyocardial pressure gradient across a coronary artery; however, in order to simplify measurement,3–7 outcome studies removed the central venous pressure from the equation, leaving Pd/Pa, thus sacrificing theoretical fidelity to create a more accessible clinical tool. Outcome studies therefore used a version of the FFR equation that had already moved away from the theoretical physiological basis of its derivation, to improve adoption in the real world.
FFR Cut-off Values
There have been two proposed cut-off values for FFR, whereby treatment decisions are decided according to whether the result lies on one side of the cut-off value or another. An FFR value of ≤0.75 was first validated against a series of non-invasive tests and represented a value that predicted a positive non-invasive ischaemia test (see Table 1). Several independent studies have since demonstrated that FFR values between 0.67 and 0.75 accurately predict positive myocardial perfusion scintigraphy, exercise treadmill and stress echo results (see Table 1).17–27 Note that, as with all in vivo tests, variability was seen across the studies. One value for FFR did not consistently predict the same thing in different populations; nor did it consistently predict response again the same test. Some of these studies also incorporated right atrial pressure, whereas others did not; this introduces a source of variability that we no longer account for in the real world. Minor variability is expected with any in vivo tool, but it is a point of potential significance when one considers the importance of a ‘cut-off’ value in clinical decision making. What appeared consistent across several studies, was that values between 0.76 and 0.80 showed sub-optimal specificity for predicting non-invasive test results and these values were, therefore, labelled as being in the ‘grey zone’, whereby clinician judgement would be required to decide whether a lesion was ischaemia-producing, based on the broader clinical picture.17–27
Despite the obvious minor variability in the measurement and predictive performance of FFR in clinical studies, it is clear from these studies that using an FFR cut-off value of ≤0.75 has a good chance of identifying ischaemia in the vessel being examined.
Percutaneous Coronary Intervention of Functionally Non-significant Stenosis
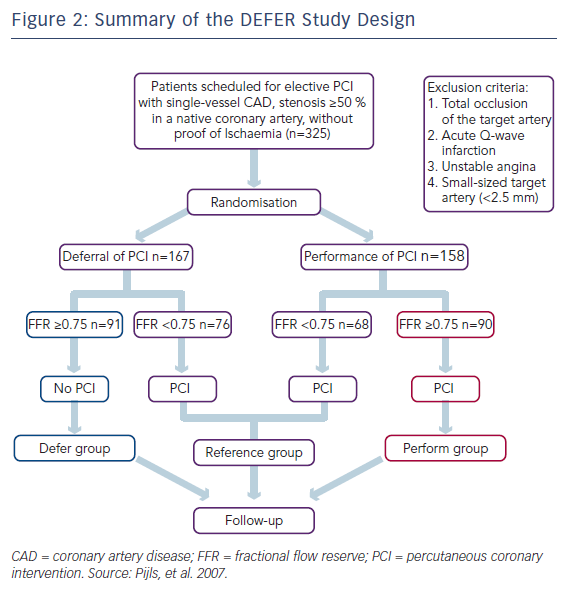
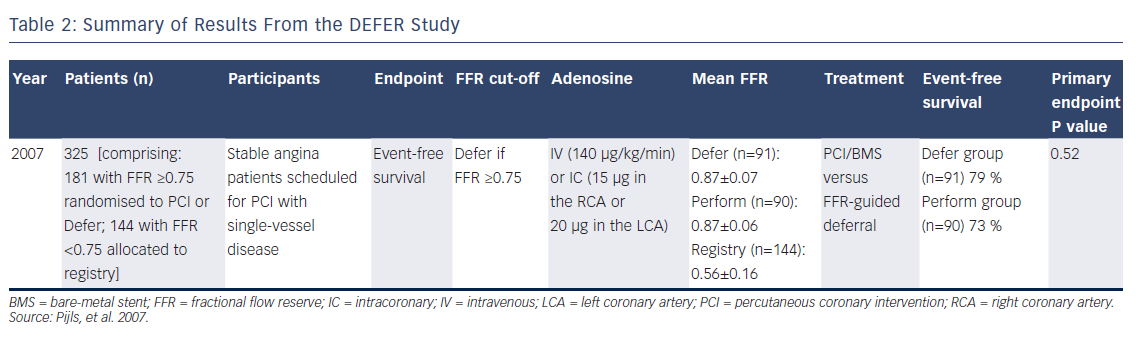
The DEFER study examined the outcomes of treating FFR-negative lesions with either stenting with percutaneous coronary intervention (PCI; Perform group) or PCI deferral (Defer group), on the basis of an FFR value of ≥0.75 (see Figure 2).3 Although the coronary interventional technologies deployed in this study were of a different era, the pattern appeared clear – stenting of lesions using PCI without a substantial pressure drop did not derive outcome benefits over 5 years of followup, and may have caused harm. Event-free survival at 5 years was 79 % in the Defer group and 73 % in the Perform group, confirming no advantage from PCI of non-flow-limiting lesions (P=0.52; see Table 2). A composite endpoint of death and MI favoured deferral in lesions with an FFR of ≥0.75.
More recent data from Korea suggest that the use of drug-eluting stents do not change this concept; there appears to be little advantage to stenting vessels that do not exhibit a significant pressure drop.28
Fractional Flow Reserve Versus Angiography for Guiding Percutaneous Coronary Intervention
In the Fractional Flow Reserve versus Angiography for Multivessel Evaluation (FAME) studies, the prognostic significance of stenting was examined in patients with multi-vessel disease who were receiving optimal medical therapy, to establish whether FFR guidance could improve outcomes. Within these studies, the FAME investigators abolished the grey zone, creating a single FFR cut-off value for predicting outcome of ≤0.80. The logic behind this, is that in identifying a patient who has ischaemia (as defined by an FFR value that would predict a positive non-invasive test), there is a certain ‘cliff-edge’ phenomenon to this result; either the patient does or does not have ischaemia at that point. However, a value that lies within a few points of this proposed cut-off value, whether currently ischaemic or not, may still serve to identify a patient at risk of progressing to events in the next few years, although data have shown that the lower the FFR value, the more likely a lesion is to lead to a clinical event during the next 5 years.29 This concept was supported by findings from a recent observational study, further confirming the gradient of risk seen within FFR values, a gradient that appeared detectable even in those within the grey zone.30
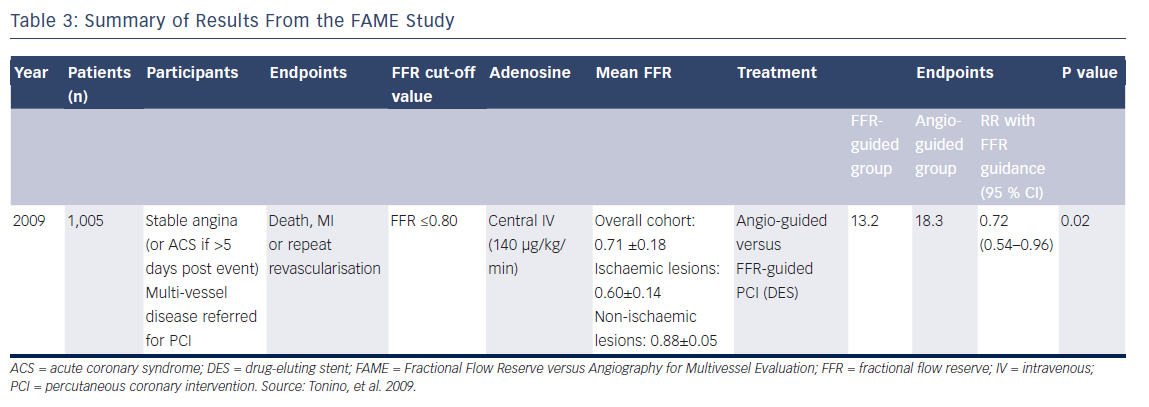
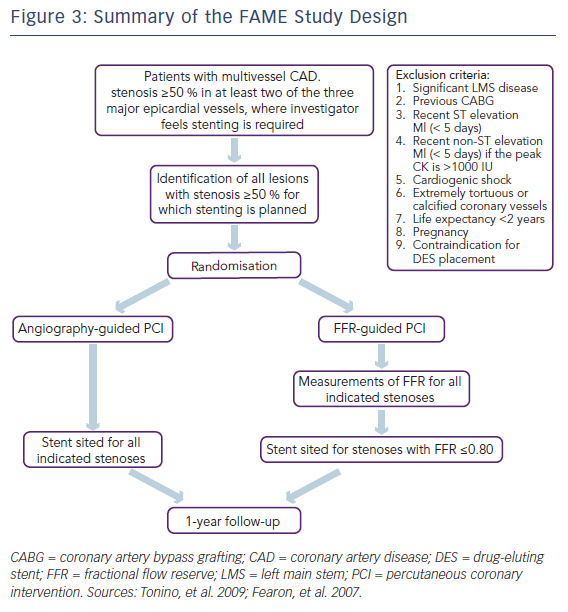
The first FAME study examined patients with multi-vessel coronary artery disease referred for PCI and performed FFR in each lesion deemed to be more than 50 % diameter luminal stenosis by visual assessment (see Figure 3).4,31 Patients were then randomised to either angiography-guided PCI, where the operator stented any lesion of >50 % severity that they deemed required PCI, or FFRguided PCI, where all lesions with an FFR ≤0.80 were treated, and the rest deferred. Better outcomes were demonstrated among the patients treated using FFR-guidance compared with those who received PCI on the basis of angiographic severity alone. The primary endpoint, a composite of death, MI and repeat revascularisation, occurred in 67 patients (13.2 %) in the FFR group and 91 patients (18.3 %) in the angiography group (RR 0.72; 95 % CI [0.54–0.96]; P=0.02; see Table 3).4
Having the COURAGE to Re-assert the Role of Percutaneous Coronary Intervention in Patients With Stable Angina
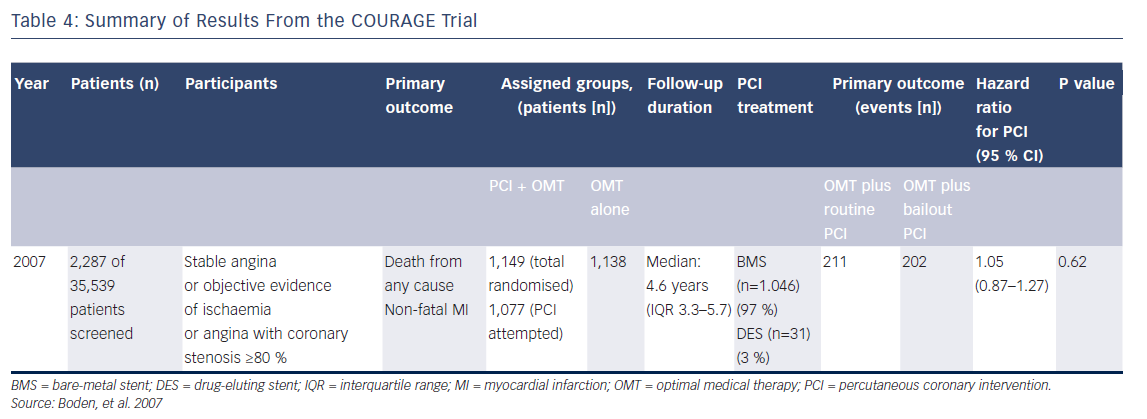
The importance of the FAME-2 study has to be set into context of the field as it existed in 2007/2008. Following the publication of the COURAGE trial results in 2007, the role of PCI in stable angina was being openly questioned.32 Comparing the event rates in IHD populations, no difference was demonstrated between the group randomised to optimal medical therapy (OMT; with bailout PCI at the investigator’s discretion) versus those randomised to PCI using bare-metal stents.
There are many important aspects of the COURAGE trial that are occasionally overlooked, which are relevant to the understanding of the importance of the FAME-2 study and the simple message that this study was then able to put forward. Importantly, in the COURAGE trial, bailout revascularisation was required in 32 % of the medical therapy group – a highly selected, low-to-medium cardiovascularrisk group of patients, randomised to receive intensive, personalised medical therapy. High rates of beta-blocker tolerance at 5 years were demonstrated in the group: 85 % in those receiving PCI plus OMT and 86 % in those receiving OMT alone (see Table 4).32 This 32 % bailout rate is not an inconsiderable failure rate for medical therapy as a primary strategy. Any mechanism that can identify these patients up front and lessen the need for bailout revascularisation would be desirable, especially in the real world, where patients tend to have lower rates of compliance with prescribed drugs.
The FAME-2 Study
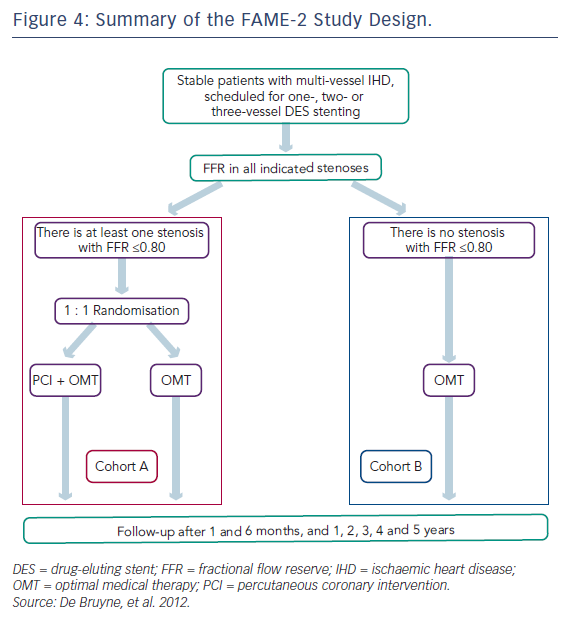
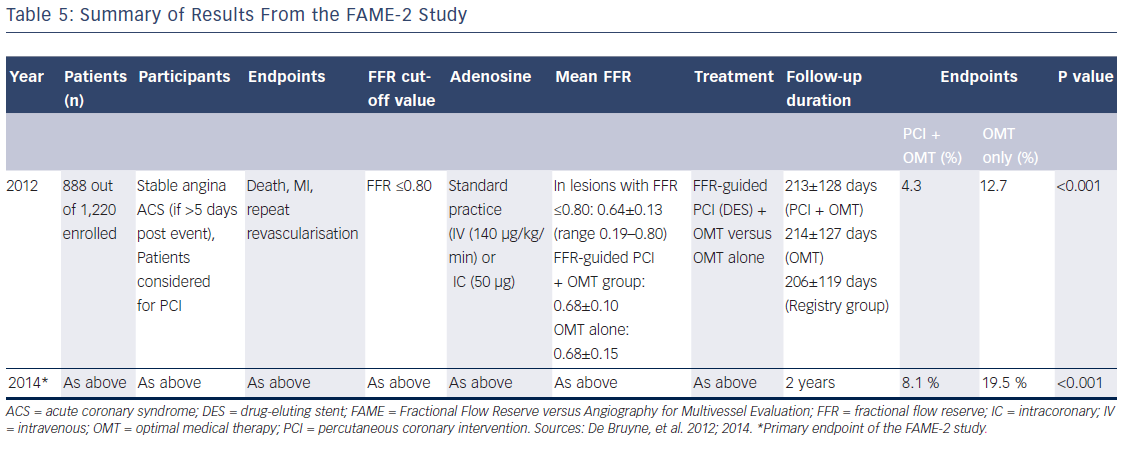
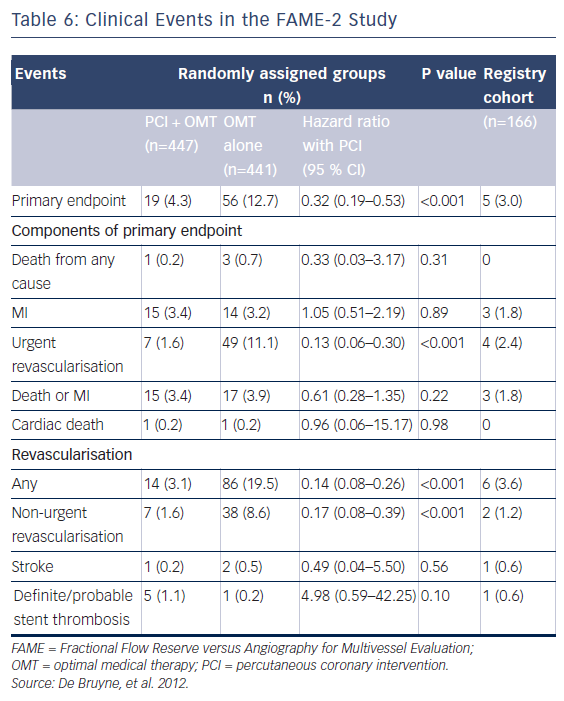
In the FAME-2 study, patients with IHD on optimal medical therapy underwent pressure wiring of all vessels with angiographically identifiable lesions.6 All pressure wire-positive patients (FFR≤0.80) were then randomised to PCI or OMT (see Figure 4). This study was stopped prematurely at a mean follow-up of 7 months due to a significant between-group difference in the incidence of the primary endpoint (4.3 % in the PCI-plus-OMT group versus 12.7 % in the OMT-alone group; hazard ratio [HR] with PCI 0.32; 95 % CI [0.19–0.53]; P<0.001; see Table 5). The primary superiority endpoint of this trial, a reduction in the rate of death, MI and urgent revascularisation, was met, principally through a reduction in the rate of urgent revascularisation. Urgent revascularisation was performed in 49 (11.1 %) patients in the OMT group versus seven (1.6 %) patients in the PCI group (HR with PCI 0.13; 95 % CI [0.06–0.30]; P<0.001; see Table 6).
The strength of this particular endpoint is often discussed. Half of these patients (n=27 [48.2 %]) had blinded adjudication committee evidence of instability (increased troponin levels, ECG changes at rest) and therefore had clear evidence of a significant acute coronary problem. In 29 patients (51.8 %) who underwent urgent revascularisation, unstable angina was diagnosed based on clinical features with no evidence of ischaemic ECG changes or positive cardiac biomarkers (see Table 7).6
Excluding the patients without objective evidence of an acute problem, the difference between the PCI group (seven patients) and the difference in patients with unstable ECGs (read by a blinded committee) or increased troponin levels (27 patients) remains significant. (P<0.001; see Table 7).6 However, there were no significant differences between the PCI group and the OMT group with regards to rates of all-cause mortality (0.2 % versus 0.7 %, respectively; P=0.31) and MI (3.4 % versus 3.2 %, respectively; P=0.89; see Table 6).
The FAME-2 study is sometimes criticised for stopping early and for the nature of the events that drove the primary endpoint. This point of view does not take account of the statistical realities of a trial looking at outcomes for stable IHD in the modern era, where ethics requires exclusion of those patients at the highest risk from their coronary disease. Because of the low likelihood of death or MI in a chronic, low- to medium-risk stable heart disease population in the modern era, the FAME-2 primary endpoint was going to be driven by the rate of unplanned revascularisation regardless of the number of randomised patients. Without a more than five-fold increase in the sample size, which would have no doubt rendered the trial financially impossible to perform, there is no way this study could have predicted death. To illustrate this fact, there were only four deaths in the trial (one in the PCI group and three in the OMT group) at the time point when the trial was stopped, having identified that the primary endpoint had been met and that this fact was highly unlikely to change. These very low death rates emphasise that in the modern era of OMT no technology would be able to predict death in low- to medium-risk patients without conducting a large trial, only to demonstrate a small actual difference in death rates. If we exclude those with high mortality risk (such as ongoing class IV angina, poor left ventricular function, left main stenosis, ‘surgical disease’, etc), we will find it hard to predict death.
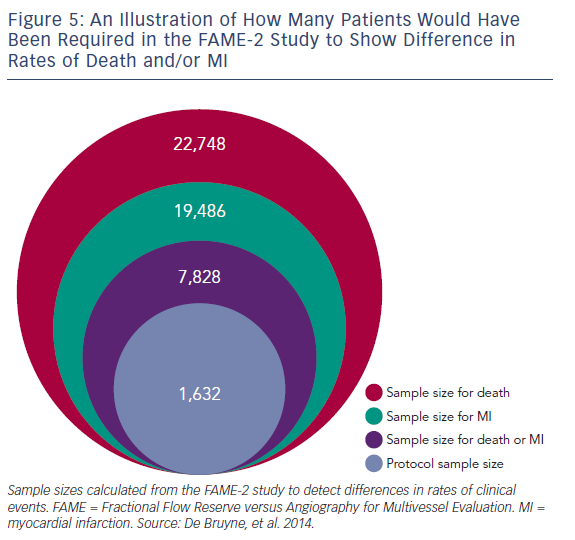
We examined the study protocol and the clinical events in the primary endpoint report from the FAME-2 study.7 The 2-year study results reported six versus eight deaths and 26 versus 30 MI events observed between the two groups. By comparing two proportions,33 we calculated the predicted sample sizes necessary to show a difference between death and MI at 2 years, when the study was originally due to report. These sample sizes were: death or MI (n=7,828), MI (n=19,486) and death (n=22,748; see Figure 5). Clearly, the sample size needed to show a difference in death/MI would have been huge and not easily possible to achieve given the cost of catheter laboratory-based trials. While the sample size for MI might be expected to fall if we take into account the different nature of MI within PCI trials (peri-procedural versus spontaneous), the sample size required would be still have to be significantly larger than that of FAME-2 study if we wanted to examine death/MI as a powered endpoint. The alternative would be a longer period of follow-up, which presumes that events will separate between the two groups after 1 year.
Summary of Fractional Flow Reserve Cut-off Values Used in Studies to Date
We have several previously proposed FFR cut-off values, each of which has been validated in selected patient populations. A cut-off of ≤0.75 has been shown to predict a positive non-invasive test with good accuracy. It has also been shown that, if one performs PCI on a patient with an FFR of ≥0.75, one is not likely to reduce the rate of death/MI (see Table 2). A cut-off value of ≤0.80 has also been shown to predict outcomes following PCI in stable disease populations with multi-vessel disease and to further improve clinical outcomes in patients on OMT.4,6
These cut-off values are clear and send a simple message to operators – that physiological guidance can help us make treatment decisions on our patients. If cut-off values are to be helpful, on a perpatient level; howeverwe need to know of any potential downsides of basing our treatment decisions on FFR results.
Potential Downsides of a Binary Cut-off Value in FFR
The clarity of the existing cut-off values for FFR helps make pressure wire technology accessible and easy to use, but questions remain regarding how to interpret these data. In order for a cut-off to have statistical and clinical power, it must clearly demarcate a difference between those above that value and those below. It must be robust, reproducible and have low false-positive and false-negative rates. After all, a binary cut-off means that if the result is 0.81, the test tells us not to perform PCI. If the result is 0.80, the test tells us to proceed. This approach may apply to particular populations, but what about the single patient in front of us? Is FFR robust enough as a test to reliably do this? Furthermore, and perhaps most importantly, are we applying our use of FFR to the same types of patients as were recruited to the key trials described above, who have been shown to benefit from this approach as a group?
Routes and Doses of Adenosine Administration
During the initial FFR validation work against non-invasive ischaemia tests, two routes of administration were used to establish cut-off values – intravenous adenosine and intracoronary adenosine.1,14,15,17–27,34–38 The adenosine doses that had diagnostic ability to identify ischaemiaproducing lesions against non-invasive tests were 140 μg/kg/min and up to maximum of 40–60 μg for intravenous administration of adenosine and intracoronary adenosine, respectively (see Table 1).
In DEFER and FAME-2 studies, operators used either intravenous or intracoronary adenosine, whereas in the first FAME study, central intravenous adenosine administration (see Tables 2, 3 and 5) was used.3,4,6,7 It is important to note that higher doses of adenosine have not been validated in clinical outcome studies and increasing the adenosine dose may alter the treatment threshold by lowering systemic blood pressure and altering the ratio between Pd and Pa (as opposed to altering the ratio by lowering peripheral microvascular resistance).
The agreement between intravenous and intracoronary adenosine is generally good at recommended doses.39,40 However, the chances of securing the same dichotomous treatment recommendation by each method of adenosine administration falls to 78 % at FFR values of approximately 0.8041 and there is a hyperaemic dose–response relationship seen with intracoronary doses of >60 μg.42 The route of adenosine administration differs from centre to centre,43 and while FFR measurement with central administration of intravenous adenosine is considered the gold standard, in the era of transradial PCI, intracoronary or peripheral intravenous administration of adenosine is more practical, although this does give similar FFR results to centrally administered adenosine.44,45
Broader FFR Limitations
FFR has several other limitations. Identification of a functionally significant coronary stenosis by FFR may be obscured by the magnitude of residual coronary microvascular resistance during hyperaemia. This can occur in the presence of high left ventricular end-diastolic pressure and/or diseases which particularly affect the microvasculature.46 Decisions based on FFR cut-off values (e.g. defer/ perform based on FFR ≤0.80) have also been shown to change once right atrial pressure is incorporated into the FFR calculation.47
Variable haemodynamic responses can also confound measurement, particularly when adenosine infusions are administered centrally48 and recorded measurements can vary according to differing operator/FFR console interpretations of minimum Pd/Pa during the hyperaemic response.49,50 Furthermore, functional significance of a stenosis may be overestimated where the vessel in question gives off important collaterals to a chronic total occlusion (CTO) vascular territory, and often normalised in a donor vessel with moderate stenosis following successful CTO PCI.51 Recanalisation of a CTO was also shown to result in a modest increase in the FFR of the predominant collateral donor vessel and was associated with a reduction in donor vessel coronary flow. As expected, a larger increase in FFR is associated with greater coronary stenosis in donor artery severity.52
These everyday factors can reclassify lesion significance where values fall near a cut-off value and may result in inappropriate treatment decisions if operators make their decisions based solely on a cut-off value.
Which Patients had Events in the Studies to Date? Were They Clustered Around the Cut-off Value?
Johnson et al. showed within a large, patient-level meta-analysis of multiple FFR trials that FFR values of <0.67 most clearly identified those at risk of MI or death.18 In fact, the mean FFR values obtained from ischaemic vessels in the DEFER, FAME and FAME-2 studies were 0.56±0.16, 0.60±0.14 and 0.68±0.10, respectively (see Tables 2, 3 and 5),3,4,6,7 which are more severe than the results we see when we use pressure wire technology in routine clinical practice to assess an intermediate stenosis of uncertain significance. Operators have frequently considered tight lesions to be more at risk of events and would not look for another test when treating such patients who have clear symptoms of angina. The available FFR trial data support that concept.
What do Existing FFR Cut-off Values Tell us About Intermediate Stenoses?
Importantly, no randomised FFR study to date has examined a cohort of angiographically intermediate lesions, where the use off FFR has been tested in the scenario of treatment uncertainty (Tables 2, 3 and 5). Despite this, many operators use FFR to assess these patients in the real world. This observation has obvious importance, as the further a value is away from a cut-off point (strongly ischaemic versus trivial obstruction), the stronger it becomes at predicting outcomes when dichotomised to PCI performance/deferral. Intermediate stenoses, however, tend to have intermediate FFR values that cluster around the cut-off point. The average FFR value in the ADVISE (Adenosine Vasodilator Independent Stenosis Evaluation) registry of intermediate coronary lesions was 0.81.53
The Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularisation (DEFINE-FLAIR) and Evaluation of iFR vs FFR in Stable Angina or Acute Coronary Syndrome (iFR Swedeheart) studies, expected to report results in late 2016/early 2017, will be the first major studies to report outcomes with FFR and instantaneous wave-free ratio (iFR) in a large (n=4,500) population of angiographically intermediate lesions.54,55 These studies will provide us with the definitive assessment of the ability of an FFR cut-off value of ≤0.80 and an iFR cut-off value of <0.90 to predict outcomes in intermediate lesions and establish whether the two modalities have similar predictive powers for future events.
If we get an FFR Value of 0.81 in an Angiographically Intermediate Stenosis, Could This Still be an Ischaemia-causing Lesion?
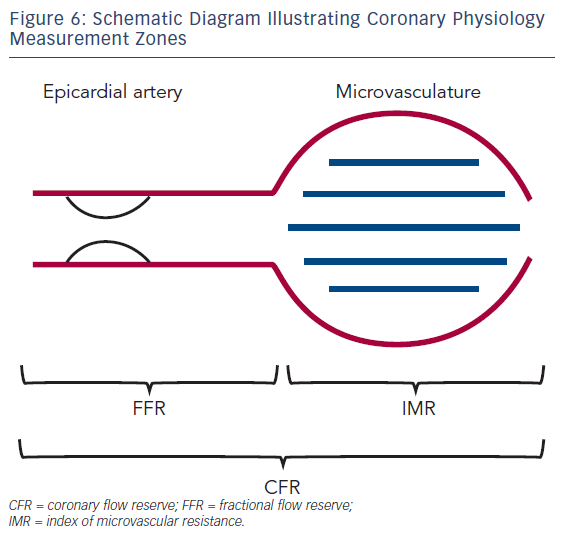
The degree of blood pressure drop across a lesion is related to the absolute flow rate across that lesion. The whole point of FFR is for an intracoronary pressure gradient to tell us what intracoronary blood flow is doing; however, in low-flow situations, a pressure drop may be underestimated and in high-flow situations, a pressure drop may overestimate the degree of obstruction.56 High blood flow creates greater friction and separation losses, causing a pressure drop, whereas low flow does the opposite, minimising observed pressure loss.57 Using Doppler-derived flow measurements, such as coronary flow velocity reserve (CFR) and the index of microcirculatory resistance, can help to identify such cases, but these techniques are time consuming and not widely available outside of a research setting (see Figure 6).
Treatment recommendation discordance (perform PCI versus deferral) between dichotomised findings derived from FFR and CFR has been reported in up to 30–40 % of coronary stenoses.58,59
Van de Hoef et al. studied 157 intermediate coronary stenoses evaluated by FFR and CFR in which revascularisation was deferred and followed-up for 11.7 years.56 Discordance between the FFR and CFR treatment recommendation occurred in 31 % and 37 % of stenoses at the 0.75 and 0.80 FFR cut-off values, respectively, and was explained by the degree of microvascular resistance during basal and hyperaemic conditions. Compared with concordant normal (‘defer’) dichotomised results of FFR and CFR, a ‘normal’ FFR with an abnormal CFR was associated with a significantly increased rate of major adverse cardiac events throughout 10 years of follow-up, regardless of the FFR cut-off applied. CFR has demonstrated abnormal flow, which is a marker of adverse outcomes and in this case, FFR has not been able to identify this finding using pressure alone. In contrast, an abnormal FFR with a normal CFR was associated with equivalent clinical outcomes when compared with normal results of both parameters; this can be explained by the fact that abnormal blood flow is one of the principal drivers of ischaemia and therefore outcomes.56
Who in Particular Might Have ‘False Positive’ Results, Whereby the FFR Value is <0.80, but who Have Good Blood Flow and low Risk?
Patients with healthy microvasculature but focal epicardial lesions can exhibit a large pressure drop across a stenosis upon introduction of adenosine. CFR studies in these patients show that the microvasculature is able to dilate such that blood flow can sometimes be increased more than three-fold across these stenoses. Of course, if a patient is able to mount a substantial increase in blood flow across an epicardial stenosis, the stenosis is not ‘flow limiting’ and is therefore unlikely to benefit from PCI as far as symtoms are concerned. Unfortunately, such patients tend to be young and have a longer lifespan to reflect on the worth of a possibly unnecessary stent procedure.
What Happens When we Dichotomise Continuous Data?
Cut-off values are artificial constructs designed to assist us in clinical decision making. However, by their nature they are conceived by analysis of groups of patients, rather than the potentially variable individual on one’s catheter laboratory table. FFR reproducibility data from the DEFER study were analysed to see the effects of FFR measurement variability on FFR-guided treatment strategy.60 This analysis showed that outside the FFR range of 0.75–0.85, classification certainty (PCI performance versus deferral) from a single FFR result is >95 %. However, closer to its cut-off value, certainty falls to <80 % within the range of 0.77–0.83; reaching a nadir of 50 % at the precisely defined cut-off point of 0.80. In clinical practice, this means that each time a single FFR value falls between the range of 0.75–0.85, there is a chance that the FFR-derived dichotomous revascularisation recommendation will change if the measurement is repeated 10 minutes later, with a 0.80 result shown to be as repeatable as a coin toss.60 This is not a limitation of FFR; rather, this is a limitation of any measured clinical variable where so many physiological factors input into the result. The consequences for the operator, however, are obvious. If we transfer all of our clinical judgement onto a single dichotomous variable, we will sometimes get a variable answer.
For a group of patients, if we subjugate our judgement to the test, we will be right considerably more often than we are wrong, whether we are looking for ischaemia or prognosis in those patients. However, this is of little comfort to the patient whose result leads to ‘treat’, when they could have been deferred, and vice versa. Fortunately, however, therefore, there remains a role for clinician judgement in the interpretation of all available variables, be they clinical, angiographic or physiological. Operators are encouraged to bear these points in mind when they use FFR, which in our view should be seen as a generally faithful servant, rather than a rigid, incontrovertible master.
Conclusion
FFR is now a staple tool in the modern catheter laboratory. Like all tests of a human physiological parameter, it has both strengths and limitations. The data on which FFR has been validated come from specific sub-groups of angiographic populations and has not yet been categorically proven in all populations. In particular, it has not yet been tested in intermediate lesions within outcome studies, although such studies are ongoing. We should therefore use the information provided by FFR as part of a broader view of our patient.
FFR cut-off values are a summation of what these studies tells us and are extremely useful in making what could be a complex tool simple and accessible to all interventional cardiologists. However, the value derived is not absolute and the dichotomous partition of a naturally variable physiologically parameter reduces the power of the information received. The lower the FFR, the more potent the predictive power for outcomes and operators should be cognisant of this finding. Basic principles tell us that a policy of simplistic absolutism is rarely in the interests of our patients and FFR is no different in that respect. We recommend widespread adoption of FFR in all modern catheter laboratories, but recommend it as an adjunct to clinical decision making, not a replacement for it, as no tool purporting to measure a human physiological parameter is perfect.