Frail originates from the French word frêle, meaning ‘of little resistance’, and from the Latin word fragilis, meaning ‘easily broken’. In medicine, frailty is a condition in which there is a decline in biological reserves and deterioration in physiological mechanisms that render a person vulnerable to a range of adverse outcomes.1 It is expected that the proportion of the world’s population over 60 years of age will nearly double from 2015 to 2050.2 Alongside this ageing population, an increased burden of frailty means that optimal clinical management of this vulnerable population is of key importance.
In recent years, there has been increasing interest in the clinical implications of frailty in patients with cardiovascular disease (CVD). There has also been an increase in the number of patients with frailty, which coexists in up to 60% of patients with CVD.3 Following stressors such as acute coronary syndrome (ACS) and invasive procedures, patients with frailty are at risk of disadvantaged health outcomes, such as dependency, disability, falls, institutionalisation and mortality.4–7 More recently, the coronavirus disease 2019 (COVID-19) pandemic has put an additional stress on these patients, emphasising the importance of frailty assessment to help individualise care for older patients with CVD.8
Frailty and disability, although interrelated, are considered distinct clinical entities. Frailty predicts disability, but disability may exacerbate frailty,5 which may lead to co-occurrence and difficulty in the assessment of frailty. As such, frailty assessment is still not routinely conducted in cardiology practice, and there is a lack of consensus on which frailty assessment tool to use and in which setting.9
This review summarises the latest evidence on common assessment tools used in people with CVD, with a particular focus on those patients with coronary and valvular diseases, and provides a synthesis of the utility of these tools in predicting outcomes in patients with CVD.
Assessment of Frailty
The concept of frailty has been described in various ways. A study identified 67 instruments that can be used to assess frailty.10 Some of these instruments focus on physical and biological aspects, whereas others focus more holistically on physical, psychological and social domains.
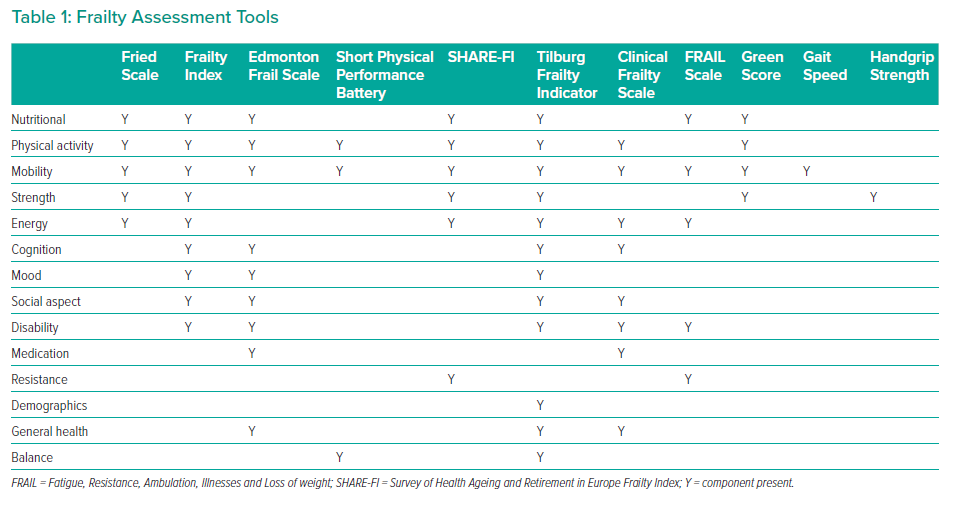
The commonly used frailty instruments discussed in this review, and the components they evaluate, are summarised in Table 1. Of note, mobility is assessed in all the multidomain tools.
Physical Frailty Phenotype or Fried’s Frailty Scale
The physical frailty phenotype, also called Fried’s frailty scale, consists of five core domains: slowness, weakness, low physical activity, exhaustion and shrinking.4 Patients meeting one or two criteria are considered as pre-frail, and those meeting three or more are considered frail. The physical frailty phenotype formed the basis of the Cardiovascular Health Study (CHS) frailty assessment and is the most frequently used instrument. Although Fried’s scale accurately predicts mortality in patients with CVD, it is not readily measurable in acute clinical situations because it includes a measurement of grip strength, a walking test and a detailed quality of life questionnaire.11
Short Physical Performance Battery
The Short Physical Performance Battery (SPPB) measures a series of three timed physical performance tests, including gait speed, chair rises and tandem balance.12 Performance on each test is scored from 0 to 4, with a total score ≤5 (of a possible 12) indicating frailty. The SPPB is relatively simple, cheap and takes approximately 10 minutes to complete. It does not require the presence of physicians, but may be difficult to administer in acute situations.
Frailty Index or Deficit Accumulation Index
The Frailty Index (FI), also known as the Deficit Accumulation Index (DAI), considers frailty across multiple domains and may include physical, psychological and social components in addition to laboratory values.13 The number of deficits identified in an individual is correlated with the level of frailty. The proportion of deficits over the number of items evaluated is expressed as a fraction, and an FI >score 0.25 is usually considered as frail.14
Survey of Health Ageing and Retirement in Europe Frailty Index
The Survey of Health Ageing and Retirement in Europe Frailty Index (SHARE-FI) is based on the Fried criteria, and evaluates exhaustion, appetite, ambulation, resistance, physical activity and handgrip strength measurement.15 The SHARE-FI is easier to measure than the original Fried scale, because the questionnaire can be easily completed at the bedside and does not require the measurement of gait speed.
Tilburg Frailty Indicator
The Tilburg Frailty Indicator is a multidimensional structured questionnaire that evaluates the physical, psychological and social domains.16 It consists of two parts. Part A has 10 questions on frailty determinants (age, sex, marital status, education level, social circumstances and lifestyle). Part B has 15 frailty elements across three domains:
- Physical, consisting of eight items (physical health, unintentional weight loss, difficulty walking and problems with balance, hearing, vision, hand strength and physical tiredness).
- Psychological, consisting of four items (cognition, depression, anxiety and coping).
- Social, consisting of has three items (living alone, social relationships and social support).
Each item in Part B scores 1 point, and patients are considered frail if they score at least 5 of a possible 15.
Clinical Frailty Scale
The Clinical Frailty Scale (CFS) was designed for the CSHA and can be readily administered in most clinical settings.17 The CFS is based on fitness, active disease, activities of daily living (ADL) and cognition, and the expanded scale ranges from 1 (very fit) to 9 (terminally ill).17,18 Because assessment relies upon the subjective judgement of a clinician, the measure is prone to interobserver variability.17
Edmonton Frail Scale
The Edmonton Frail Scale (EFS) is another multidimensional scale, comprising 10 domains with 17 potential deficits covering cognition, general health status, functional independence, social support, medication use, nutrition, mood, continence and functional performance.19
The EFS includes the clock test for assessment of cognitive impairment, and the Timed Get Up and Go (TUG) for balance and mobility. The cut-off point for frailty is 12 or more deficits. The EFS has good correlation with the opinion of a specialist following a Comprehensive Geriatric Assessment (CGA).19 Because a CGA is time consuming, the EFS offers a rapid screening tool for the non-geriatric specialist.
Reported Edmonton Frail Scale
The Reported Edmonton Frail Scale (REFS) includes nine frailty domains: cognition, general health status, functional independence, social support, medication use, nutrition, mood, continence and functional performance.20 Compared with the EFS, the REFS is based on self-reported functioning, and is appropriate in patients able to complete a questionnaire. Frailty is identified by a score of at least 8.
Hospital Frailty Risk Score
The Hospital Frailty Risk Score (HFRS) uses ICD-10 diagnostic codes from electronic healthcare records to identify frailty. It includes more than 100 variables derived from routinely collected data and has been validated against both the Fried scale and other FI measures.21
Fatigue, Resistance, Ambulation, Illnesses and Loss of Weight Scale
The Fatigue, Resistance, Ambulation, Illnesses and Loss of weight (FRAIL) scale is a brief, interview-based screening tool. The FRAIL scale is commonly used in the acute setting because it does not include items that are difficult to measure (e.g., walk speed, handgrip strength, stand-up test).22
Comprehensive Geriatric Assessment
The CGA is considered to be the gold standard for frailty assessment and involves a holistic, multidimensional and interdisciplinary assessment of an individual, culminating in the formulation of an individualised management plan.23 The CGA is time consuming and is not part of the routine care of older people. Potentially useful brief screening tests include measuring 5 m gait speed, which is highly predictive of cardiovascular mortality, or handgrip strength.24–27 These frailty assessment tools are all different. Some scales, such as the FRAIL scale, FI and CFS, are based on interviews without objective assessment of physical performance and have a prognostic implication in patients with ACS.28
Frailty and Cardiovascular Disease
The association between frailty and CVD is bidirectional, because frailty is associated with an increased risk of CVD and CVD mortality,11,29 and CVD is associated with an up to threefold increase in frailty.3,30,31 Insights from the CHS have shown that subclinical CVD measures strongly predict frailty, even after adjustment for traditional CVD risk factors, whereas being overweight or obese and having a higher age-adjusted composite coronary artery score in midlife were associated with frailty 26 years later.32,33 This implies that frailty and CVD may also have long-term connections that should be recognised.
A meta-analysis by Veronese et al. of 31,343 patients from 18 studies evaluated the prevalence and incidence of CVD according to frailty status.11 Most of the patients were community-dwellers in Europe. Veronese et al. found that frailty and prefrailty were associated with a greater chance of having CVD, with adjusted ORs of 2.85 (95% CI [2.29–3.53]) and 1.63 (95% CI [1.39–1.91]), respectively.11 Frailty, which was found in 17.9%, was associated with an increased risk of CHD, heart failure and risk of cardiovascular mortality, whereas prefrailty carried a higher risk of heart failure and cardiovascular mortality.11 Supplementary Table 1 summarises studies assessing the outcomes of patients with frailty and CVD.
Three components of a modified Fried scale, namely low energy expenditure (p=0.03), exhaustion (p=0.01) and slow gait speed (p=0.03), were shown to be significantly associated with CVD onset, whereas two were not (unintentional weight loss and weakness).34 An independent association was demonstrated between prefrailty and the development of CVD, with low gait speed the best predictor of future CVD. The risk was higher in those meeting two frailty criteria (HR 1.79; 95% CI [1.27–2.52]) rather than one (HR 1.25; 95% CI [1.05–1.64]).34
A limitation in physical functioning alone appears to be associated with a range of important clinical outcomes. Newman et al. used an extended walking test (400 m) to assess frailty in 3,075 community-dwelling adults, 86% of whom completed the test.29 Compared with the quartile with the fastest walk time (<290 s), the quartile with the slowest walk time (>362 s) had a significantly higher adjusted risk of mortality (HR 3.23; 95% CI [2.11–4.94]), CVD (HR 1.61; 95% CI [1.05–2.45]), mobility limitation (HR 4.43; 95% CI [3.39–5.78]) and disability (HR 4.43; 95% CI [2.88–6.82]).29 A comparison of gait speed and 6-minute walk distance in 1,474 older people with CVD found that both were associated with all-cause mortality (adjusted HR per 0.1 m/s increase in gait speed 0.87, 95% CI [0.81–0.93], p<0.001; adjusted HR per 10 m increase in 6-minute walk distance 0.96, 95% CI [0.94–0.97], p<0.001).27
In a secondary analysis of longitudinal data, 35 instruments were grouped into four domains, namely Fried phenotype, multidimensional, accumulation of deficits and disability.35 The authors of that study showed that multidimensional frailty scores may have a stronger and more stable association with all-cause mortality and the incidence of cardiovascular events.35 A comparison of a 48-item cumulative deficit index (DI) and a phenotypic frailty index (PFI) showed that death was significantly better predicted by the DI (relative risk [RR] 1.035; 95% CI [1.026–1.045]) than the PFI (RR 1.014; 95% CI [1.009–1.019]) when calculating risks attributable to a 1% increase in the respective index.36 This may be explained by the inclusion of a broader spectrum of disorders and a greater number deficits in the DI than PFI.
Frailty and Acute Coronary Syndrome
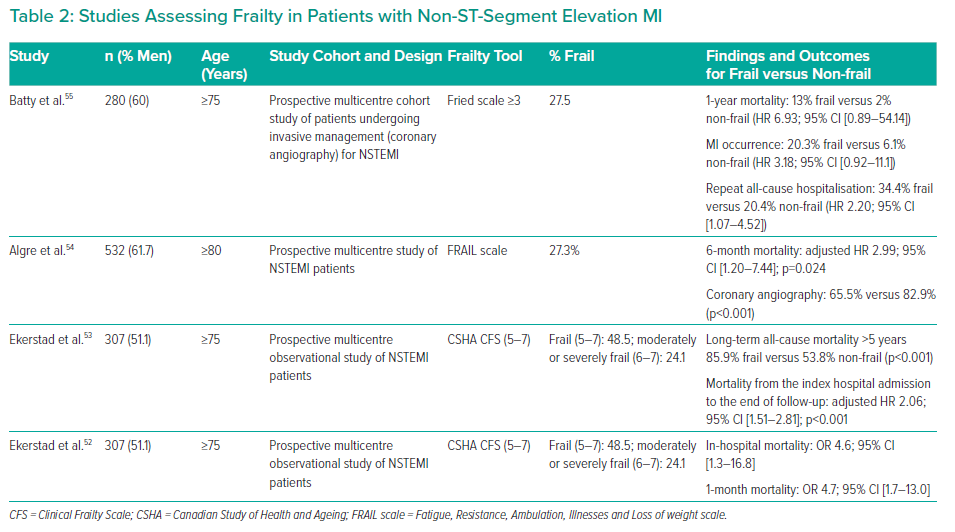
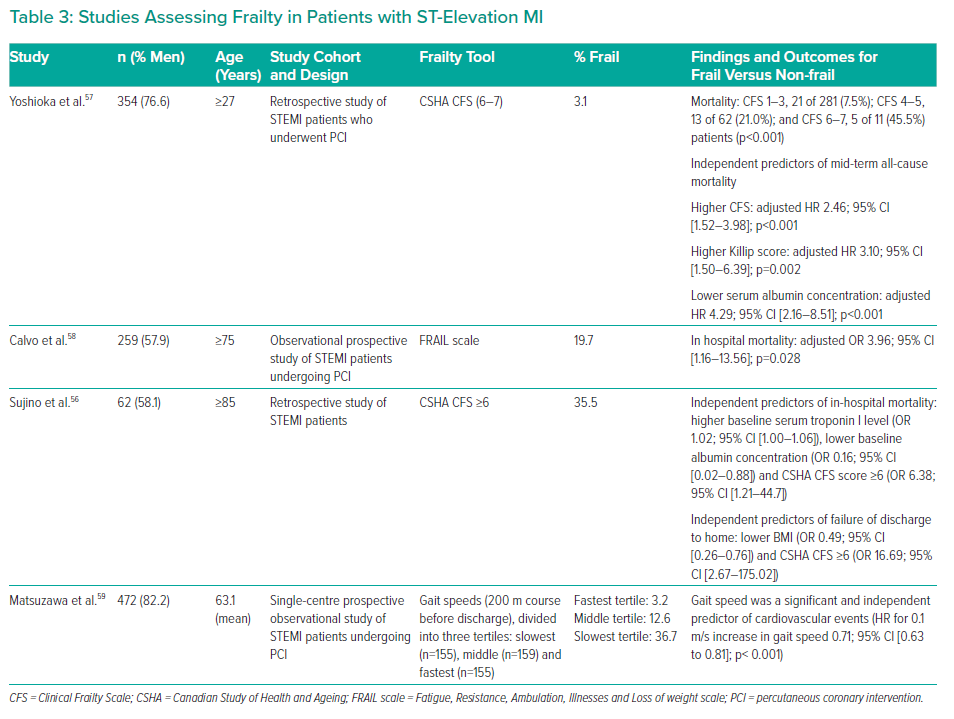
The relationship between frailty and the risk of adverse outcome following ST-segment elevation MI (STEMI) and non-STEMI (NSTEMI) has been demonstrated by many studies using different frailty assessment tools (Supplementary Table 2, Table 2 and Table 3). A recent systematic review and meta-analysis evaluated the prognostic value of frailty in 8,554 patients with ACS.37 Frailty was associated with a several-fold increase in the adjusted risk of mortality for patients with STEMI (HR 6.51; 95% CI [2.01–21.10]) and NSTEMI (HR 2.63; 95% CI [1.51–4.60]). A higher risk of mortality was also demonstrated in patients with prefrailty (adjusted HR 1.41; 95% CI [1.19–1.66]).
Blanco et al. evaluated the association between frailty and mortality in 236 people aged ≥80 years with ACS (32.2% STEMI, 67.8% NSTEMI).38 The frailest group (EFS >7) comprised 20.8% of the cohort and had the lowest survival rate after a mean follow-up duration of 470 days (38.8% versus 82.4% for the least frail group). Frailty was significantly and independently associated with an increase in the risk of all-cause mortality for the frailest compared with non-frail group (adjusted HR 4.03; 95% CI [2.02–8.04]). Graham et al. also used the EFS for 183 patients with ACS (19.1% STEMI, 80.9% NSTEMI), but in a younger cohort (age ≥65 years).39 In that study, 30.1% of patients had an EFS of ≥7, and these individuals had the highest 1-year mortality (12.7% versus 7.7% and 1.6% for EFS 4–6 and 0–3, respectively). After adjustment for baseline risk differences, the risk of 1-year mortality was 3-5-fold higher for those with an EFS ≥7 than those with an EFS of 0–3 (HR 3.49; 95% CI [1.08–7.61]).39
In another study on patients with ACS (37% STEMI, 41% NSTEMI, 21.9% unstable angina), 48.1% were frail according to the REFS.40 Fewer patients with frailty underwent percutaneous coronary intervention (PCI) than those without frailty (41.7% versus 58.3%; p=0.003). After a 30-day follow-up, frailty was significantly associated with increased risk of arrhythmias during hospitalisation (adjusted OR 2.24; 95% CI [1.32–3.80]), hospital-acquired pneumonia (adjusted OR 2.27; 95% CI [1.24 4.17]), in-hospital mortality (adjusted OR 3.02; 95% CI [1.35–6.75]), 30-day mortality (adjusted OR 3.28; 95% CI [1.59–6.76]) and 30-day readmission (adjusted OR 2.53; 95% CI [1.38–4.63]), suggesting that REFS is a useful tool for identifying patients that are at risk of a poor prognosis in the short term.
A study of 234 patients with ACS (37.1% STEMI) found that 40.2% of participants were frail according to their SHARE-FI score.15 Frailty was independently associated with a composite of death, non-fatal MI or major bleeding (adjusted HR 2.14; 95% CI [1.13–4.04]) and hospital readmission (adjusted HR 1.80; 95% CI [1.00–3.22]).15 A study using an FI based on claims data in patients with MI found that 19% were frail, and that frailty was associated with 25% greater in-hospital mortality (adjusted OR 1.25; 95% CI [1.22–1.28]).41 Interestingly, although patients with frailty were less likely to receive invasive interventions such as PCI and coronary artery bypass grafting (CABG), their hospital mortality was lower if they had these interventions rather than having none (OR 0.59, 95% CI [0.55–0.63] for PCI; OR 0.77, 95% CI [0.65–0.93] for CABG).
In the TRILOGY ACS trial, frailty was evaluated in 4,996 patients with unstable angina or NSTEMI randomised to clopidogrel or prasugrel.42 The primary endpoint was a composite of cardiovascular death, MI or stroke over 30 months. Frailty was identified in 4.7% of participants using the Fried scale and was independently associated with the primary endpoint (frail versus not-frail: adjusted HR 1.52, 95% CI [1.18–1.98]). There was no significant association between frailty and bleeding (adjusted HR 0.63; 95% CI [0.15–2.58]).42
A study of 7,398,572 patients with ACS (66.8% NSTEMI or unstable angina, 33.2% STEMI) used the HFRS based on ICD-9 codes and divided patients into three frailty groups: low-risk score (LRS), intermediate-risk score (IRS) and high-risk score (HRS).43 In that study, 0.1% of patients were classified as HRS, and these patients had significantly more bleeding complications (OR 2.34; 95% CI [2.03–2.69]), vascular complications (OR 2.08; 95% CI [1.79–2.41]), in-hospital stroke (OR 7.84; 95% CI [6.93–8.86]) and in-hospital mortality (OR 2.57; 95% CI [2.18–3.04]) than patients classified as LRS. Patients with HRS were more likely to be managed medically without coronary angiography (31.0%, 54.8% and 70.9% in the LRS, IRS and HRS groups, respectively) and less likely to undergo PCI (42.9%, 21.0% and 14.6% in the LRS, IRS and HRS groups, respectively). Among those who underwent PCI, HRS patients had higher adjusted odds of in-hospital death (OR 9.91; 95% CI [7.17–13.71]), bleeding (OR 4.99; 95% CI [3.82–6.51]) and vascular injury (OR 3.96; 95% CI [3.00–5.23]) than LRS patients.44
Sanchis et al. used the Fried and Green (uses serum albumin, Katz ADL, gait speed and grip strength) scores to assess frailty in patients with ACS (21% STEMI, 79% NSTEMI or unstable angina) at discharge and evaluated post-discharge mortality at a median follow-up of 30 months.45 Frailty when assessed with the Green score demonstrated strongest discriminative accuracy (area under curve [AUC] 0.76) for mortality. A Green score ≥5 was the strongest predictor of mortality (HR 3.4; 95% CI [1.8–6.2]) and death or MI (HR 1.8; 95% CI [1.2–2.8]). Conversely, the Fried score (≥3) was not predictive of mortality (p=0.4) after adjusting for Green score.45 This backs up a recent study, which found sex differences using the Fried score in 488 ACS patients (79.1% NSTEMI).46 A Fried score of ≥3 and the Fried score along its continuum (per 1-point increase) were independently associated with a higher risk of death in the whole sample, but these results were different between men and women. In men, a Fried score of ≥3 was independently associated with all-cause death (HR 1.89; 95% CI [1.25–2.85]), but this relationship was neutral in women (HR 0.92; 95% CI [0.57–1.49]).46
In a comparison study of seven frailty scales for patients admitted for ACS (33% STEMI, 45% NSTEMI, 22% unstable angina), Campo et al. measured the risk of major adverse cardiovascular and cerebrovascular events (MACCE) and all-cause mortality at 1 year.47 The SPPB, EFS and Fried scales were associated with all-cause mortality, but the SPPB was found to be the best predictor for MACCE (ΔC-statistic: 0.043) and all-cause mortality (ΔC-statistic: 0.063).
Using the CFS for 352 patients with ACS (STEMI and NSTEMI), Kang et al. found that frailty was strongly and independently associated with all-cause mortality (HR 5.393; 95% CI [1.477–19.692]) and unscheduled return visit (HR 2.832; 95% CI [1.140–7.037]).48 Frail patients were also less likely to undergo coronary angiography (75.66% versus 85.0%; p=0.027). Haemoglobin, albumin and prealbumin concentrations were all significantly lower, whereas high-sensitivity C-reactive protein and interleukin-6 were significantly higher, in frail compared with non-frail patients.48 In another study using CFS (≥5), 11% of the 745 patients with either stable angina or ACS (39.6% STEMI) undergoing PCI were frail.49 In that study, the authors demonstrated a significant association between frailty and increased 30-day mortality (HR 4.8; 95% CI [1.4–16.3]), 1-year mortality (HR 5.9; 95% CI [2.5–13.8]) and longer hospitalisation after PCI.
Similar findings were reported by another study on 629 patients who underwent PCI for coronary artery disease (CAD) but in whom frailty was assessed using the Fried scale.50 The association of frailty with mortality or MI at 3 years was significant (HR 2.74; 95% CI [1.12–6.71]) and more prevalent compared with non-frail patients (28% versus 6%). In addition, frailty, comorbidity measured on the Charlson Index and quality of life measured by the 36-Item Short Form Health Survey (SF-36) were associated with adverse long-term outcomes after PCI, and all significantly improved the prognostic ability of the Mayo Clinic risk score.50
Furthermore, using the Fried scale on patients with CAD undergoing PCI (11.9% STEMI, 15.4% NSTEMI) did not reveal any significant differences in 30-day outcomes (death, MI and revascularisation).51 However, the authors of that study demonstrated that the 18.6% of patients who were frail had poorer health status than non-frail patients using the SF-36 and Seattle Angina Questionnaire, and that they had more multivessel or left main CAD than intermediate frail and non-frail patients (74% versus 68% and 60%, respectively; p=0.019).
Frailty and NSTEMI
Table 2 lists studies assessing frailty in patients with NSTEMI. One prospective multicentre observational study of 307 patients with NSTEMI found that 48.5% were frail (CFS 5–7).52 Frailty was strongly and independently associated with in-hospital mortality (OR 4.6; 95% CI [1.3–16.8]) and 1-month mortality (OR 4.7; 95% CI [1.7–13.0]). At the 5-year follow-up, patients with frailty had significantly higher all-cause mortality than patients without frailty (85.9% versus 53.8% [p<0.001]; adjusted HR 2.06; 95% CI [1.51–2.81]). 53
Similarly, the FRAIL scale was used to screen for frailty in 532 patients aged ≥80 years with NSTEMI.54 Both frailty and prefrailty were associated with 6-month mortality compared with patients without frailty (adjusted HR 2.99, 95% CI [1.20–7.44] for frailty; adjusted HR 2.71, 95% CI [1.09–6.73] for prefrailty). Coronary angiography was performed in fewer patients with than without frailty, as reported elsewhere.52
The ICON1 study used the Fried criteria to classify 280 patients with NSTEMI from two tertiary centres undergoing invasive treatment strategy, and found 27.5% were frail.55 The primary outcome, which was a composite of MI, need for urgent repeat revascularisation, stroke, significant bleeding and all-cause mortality at 1 year, occurred in more frail than robust patients (39% versus 18%; HR 2.79; 95% CI [1.28–6.08]). After 1 year, mortality was more common in those with frailty compared with the robust group (13% versus 2%; HR 6.93; 95% CI [0.89–54.14]), as was the occurrence of MI (20.3% versus 6.1%; HR 3.18, 95% CI [0.92–11.1]).
Frailty and STEMI
Sujino et al. studied early outcomes in 62 patients aged ≥85 years with STEMI (67.7% underwent primary PCI; the rest received conservative therapy).56 According to the CSHA CFS (≥6), 35.5% of patients were frail. Sujino et al. found that higher baseline serum troponin I concentrations (OR 1.02; 95% CI [1.00–1.06]), lower baseline albumin concentrations (OR 0.16: 95% CI [0.02–0.88]) and a CSHA CFS score ≥6 (OR 6.38; 95% CI [1.21–44.7]) were independent predictors of in-hospital mortality. Lower BMI (OR 0.49; 95% CI [0.26–0.76]) and CSHA CFS ≥6 (OR 16.69; 95% CI [2.67–175.02]) were identified as independent predictors of failure of discharge to home.56
An association between severe frailty and mid-term mortality was also observed in STEMI patients undergoing PCI.57 In that study, 3.1% of the 354 patients were frail according to the CFS (≥6), and this was identified as an independent predictor of mid-term all-cause mortality (adjusted HR 2.46; 95% CI [1.52–3.98]), together with higher Killip score (adjusted HR 3.10; 95% CI [1.50–6.39]) and lower serum albumin concentrations (adjusted HR 4.29; 95% CI [2.16–8.51]).
Furthermore, Calvo et al. found higher in-hospital mortality for frail STEMI patients undergoing PCI (adjusted OR 3.96; 95% CI [1.16–13.56]).58 In that study, 19.7% of the 259 patients were classified as frail using the FRAIL scale. This predictive model of a simple geriatric assessment showed an optimal ability for predicting in-hospital mortality (AUC 0.83) in patients undergoing PCI.
In addition to mortality, cardiovascular events in STEMI patients undergoing PCI can be predicted using gait speed (HR for 0.1 m/s increase in gait speed: 0.71).59 This shows the variety of frailty tools that can be used to predict worse outcomes in ACS patients and those undergoing invasive interventions. Table 3 summarises studies assessing frailty in patients with STEMI.
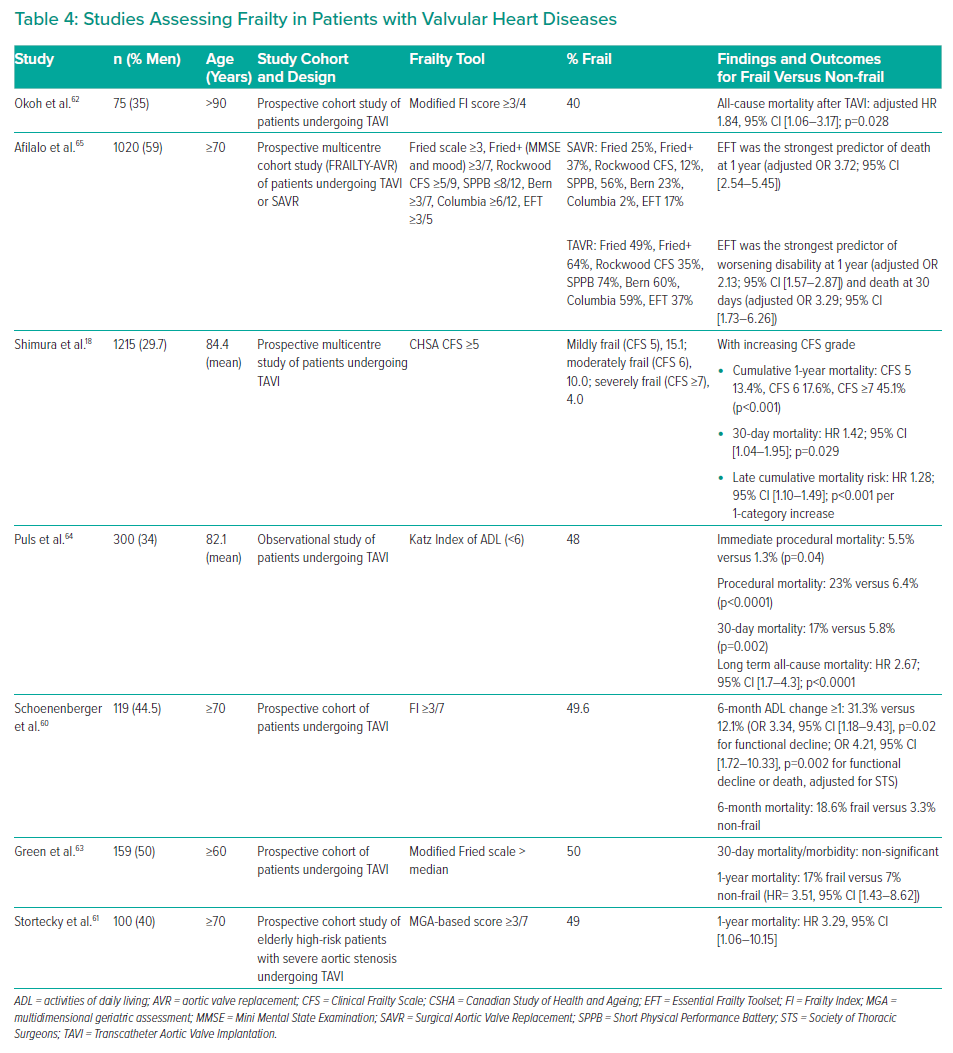
Frailty and Valvular Heart Disease
Frailty, as assessed by an FI based on assessment of cognition, mobility, nutrition and instrumental and basic ADL, has shown to be highly predictive of functional decline in older people undergoing transcatheter aortic valve implantation (TAVI).60 Worse outcomes were also demonstrated in the 49% of patients who were identified as frail using a multidimensional geriatric assessment (MGA) consisting of Mini Mental State Examination, mini nutritional assessment, TUG, basic ADL, instrumental ADL and a preclinical mobility disability.61 A higher score on this MGA-based assessment tool was associated with all-cause mortality and MACCE in this cohort, with ORs for 1-year mortality for MGA compared with Society of Thoracic Surgeons score and EuroSCORE of 3.68 (95% CI [1.21–11.19]) and 3.29 (95% CI [1.06–10.15]) on univariate and bivariable analysis, respectively.
Similarly, Okoh et al. assessed ADL as well as handgrip strength, gait speed and serum albumin for frailty in patients undergoing TAVI.62 High frailty status, defined as meeting three or four of the criteria, was an independent predictor of increased all-cause mortality (adjusted HR 1.84; 95% CI [1.06–3.17]). In another study on 1,215 patients undergoing TAVI, CFS grade increment was also found to be significantly associated with a 42% higher 30-day mortality (HR 1.42; 95% CI [1.04–1.95]).18
In a study by Green et al., 50% of the 159 patients were frail according to a modified Fried scale.63 Frailty was associated with increased 1-year mortality after TAVI (HR 3.51; 95% CI [1.43–8.62]). Interestingly, gait speed and grip strength, which are both part of the Fried scale, were not associated with survival after TAVI. Instead, ADL status measured with the Katz ADL survey and serum albumin were better than gait speed for identifying frailty-related risk after TAVI.63 These findings are concordant with a similar study, in which a low Katz Index (<6) was considered to be an independent predictor of long-term all-cause mortality (HR 2.67; 95% CI [1.7–4.3]) after TAVI.64
In a comparative study including the Fried scale and the CFS, the Essential Frailty Toolset (EFT) was most clearly associated with adverse outcomes, including 1-year mortality (adjusted OR 3.72; 95% CI [2.54–5.45]), worsening disability at 1 year (adjusted OR 2.13; 95% CI [1.57–2.87]) and death at 30 days (adjusted OR 3.29; 95% CI [1.73–6.26]) in patients undergoing TAVI (63.3%) or surgical aortic valve replacement (36.7%).65 The EFT is comprised of four items: lower-extremity weakness, cognitive impairment, anaemia, and hypoalbuminaemia. Table 4 summarises the studies assessing frailty in patients with valvular heart diseases.
Discussion
It is increasingly recognised that frailty assessment has the potential to contribute valuable prognostic information in order to inform shared decision making in patients with CVD.9 However, the translation from research to clinical practice remains a challenge, and consensus is lacking on the best tool to use in routine clinical practice.66 This review has summarised the features of frailty instruments used in cardiovascular studies and their utility in clinical practice. It also provides a detailed analysis of outcomes in patients with CVD, with a particular emphasis on coronary and valvular heart diseases.
The most appropriate tool to use is clearly setting dependent, although most frailty scores were developed in the community population. For example, the FI tends to be more commonly used in clinical research datasets, although this has been successfully implemented into routine clinical practice using electronic primary healthcare records.67,68 This has the advantage of enabling the estimation of a ‘baseline’ frailty state, calculated before an acute presentation. However, more accurate assessment is required because the ‘baseline’ frailty state may be independent of the clinical state at the time of hospital admission.
Frailty is associated with both CVD mortality and non-CVD mortality, which highlights the importance of considering the competing risk of non-CVD mortality when assessing the benefit of CVD interventions in clinical practice.9,69 This is particularly important in a population that is at particular risk of iatrogenic harm. However, the implementation of multidimensional or complex assessment tools, although accurate at predicting mortality in CVD patients, can be challenging in time-dependent situations.35,36,70 Options for frailty assessment in the clinical setting include performance tests that assess the physical functioning of patients. Epidemiological data suggest that slow gait speed is the first domain of the frailty phenotype to manifest rather than weight loss, which tends to occur at a later stage, and the use of gait speed reliably identifies patients at risk of cardiovascular events and mortality.27,29,34,59,71 A decrease in physiological reserve when evaluating physical functioning, or the presence of multisystem deficits, gives useful data on likely recovery after a stressor event, such as ACS or an invasive procedure.60
The assessment of frailty on the Fried scale and EFS has been adapted in many studies evaluating patients with ACS and has consistently been associated with mortality.38,39,42,45,47 However, the use of the Fried scale has been questioned in recent studies that found it inferior to other scales or different between sexes, although further analysis is needed to provide a definite answer.45,46 This shows there is no consensus as to which frailty assessment tool to use even though different studies evaluated similar outcomes. In these cases, the use of a well-established tool in the hospital setting may be recommended. However, in studies on patients with NSTEMI or STEMI undergoing PCI, the use of CFS and the FRAIL scale is more commonly seen, suggesting that their use seems more accepted in acute interventional cardiology, although further comparative studies are required to provide a better assessment.54,56–58
The ease and speed with which these assessments can be completed makes their use appealing. If these frailty scales consistently demonstrate reproducibility and efficacy at predicting outcomes, they could be considered as an ideal frailty instrument. There is also a dearth of evidence on the risk of cardiac interventions instead of medical management in patients with frailty. Although invasive interventions are associated with poor outcomes in patients with frailty, this should be weighed against the risk of not intervening, which may result in poorer quality of life with repeated hospitalisation. In these circumstances, frailty assessment for informed decision making requires more clarity.
Alternatively, a multidimensional assessment of frailty may be needed. Social frailty was positively associated with physical frailty on the CHS score, whereas the FI and EFS have proved to most accurately predict mortality in comparative studies.72,73 Other frailty subtypes, such as nutritional frailty, may also have a crucial role in predicting outcomes in CVD patients.74,75 Worse quality of life has also been linked to frailty, which supports the importance of multidimensional assessment tools.76 Many studies evaluating frailty in patients undergoing TAVI have included ADL as well as the use of serum biomarkers and assessment of physical frailty.61,62 ADL have even been shown to be better than gait speed at predicting survival in TAVI patients, and may offer potential prognostic aspects in this setting.63 However, the use of ADL or the DAI may not be fully representative of frailty, but partly an element of disability.
Future Directions
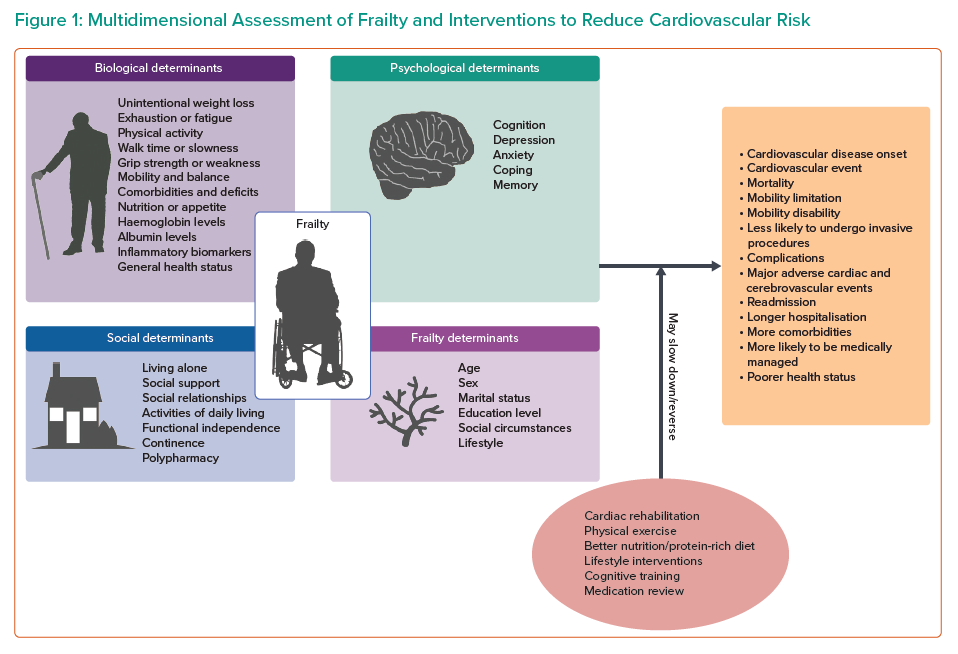
Figure 1 summarises the different components present in the assessment of frailty in the different frailty assessment tools discussed in this review. It has been suggested that the progression to frailty may be slowed, which could potentially lead to better outcomes. Suggested interventions in older patients with frailty include increased physical activity, cardiac rehabilitation, a protein-rich diet, cognitive training and medication optimisation.77–81 In addition, an exercise intervention has shown promising results in older patients after MI.82 However, evidence is lacking that these measures have a significant effect on overall trajectory, especially in patients with CVD, and further investigation is certainly warranted in this area.
Conclusion
Frailty is common among patients with CVD and is associated with disadvantaged clinical outcomes. Knowledge of a person’s frailty status provides valuable information on prognosis that may be useful in guiding informed shared decision making regarding treatment strategy. The frailty scales discussed are all useful, and personal preference and ease of implementation will play a role as to which one to use. Although the Fried criteria and FI are the most commonly used tools in research, perhaps the use of an easy and quick scale, such as the CFS and FRAIL, or one based on routinely collected data, such as the FI or HFRS, may be more feasible in clinical practice. Currently, however, there is no agreement on the optimal frailty assessment tool, and research into whether decision making based on the routine assessment of frailty improves patient outcomes in cardiology practice is ongoing.
Click here to view Supplementary Material.