Previous preclinical work demonstrated left ventricular (LV) unloading initiated at least 30 minutes prior to reperfusion reduces LV wall stress and infarct size, despite delaying reperfusion by 60 minutes in acute MI (AMI). Dr Swain’s recent publication showed that transvalvular unloading by Impella and delayed reperfusion preserve myocardial energy substrate utilisation, ultimately protecting mitochondrial function in AMI.1 In a comparison of Impella and venoarterial extracorporeal membrane oxygenation, only unloading by Impella was able to reduce infarct size by protecting mitochondrial function.1 To understand the underlying mechanism behind the benefits of initiating unloading during the ischaemic phase, Dr Swain and her team set out to differentiate the molecular changes in ischaemia from those during reperfusion.
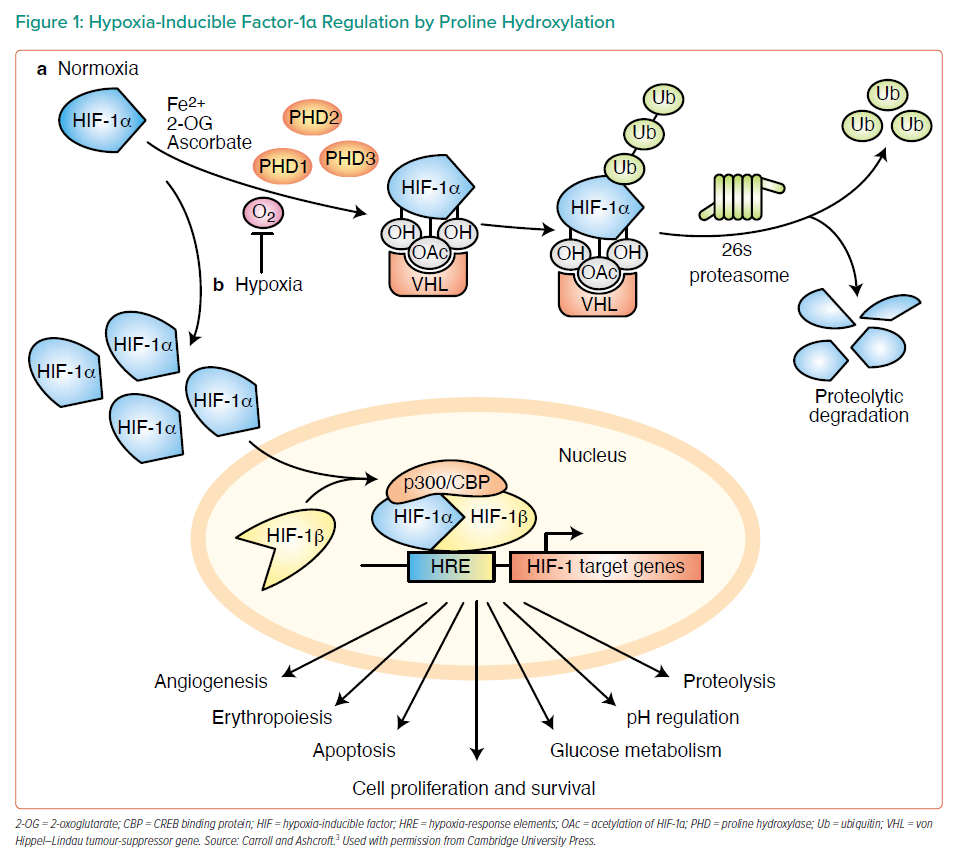
Dr Swain provided a brief overview of the major molecular mechanisms in ischaemia versus reperfusion, emphasising that in ischaemia cells switch to anaerobic glycolysis by upregulating the expression of genes involved in anaerobic metabolism.2 A key consideration in ischaemia versus reperfusion is the molecular mechanism for switching to anaerobic glycolysis when oxygen is absent. Hypoxia-inducible factor-1α (HIF-1α) is critically involved in the molecular sensing of oxygen. In the presence of oxygen, prolyl hydroxylase domain (PHD) enzymes hydroxylate HIF-1α, facilitating recognition of HIF-1α by von Hippel–Lindau tumour-suppressor protein, leading to ubiquitination and proteolytic degradation. Under hypoxic conditions, HIF-1α accumulates in the cytoplasm, translocates to the nucleus and forms complexes that trigger upregulation of target genes involved in glycolysis (Figure 1).3
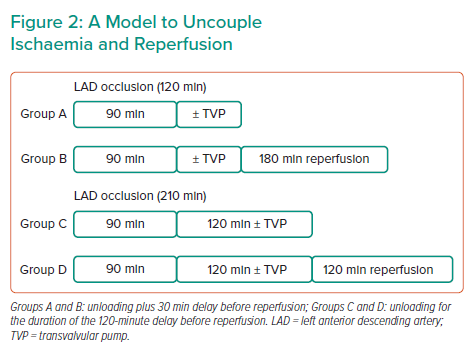
Dr Swain and her team designed a model to uncouple ischaemia and reperfusion (Figure 2). Four experimental groups were tested. Groups A and B were exposed to shorter ischaemia of 120 minutes; after 90 minutes ischaemia, Group A received unloading with a transvalvular pump for 30 minutes and was terminated without reperfusion, whereas Group B was reperfused for an additional 3 hours after the 30-minute unloading. Groups C and D were exposed to a prolonged ischaemic time of 210 minutes to mimic the real-life delay between patient symptoms and balloon time: after 90 minutes ischaemia, Group C remained in ischaemia for 120 minutes with unloading, whereas Group D subsequently received 2 hours reperfusion. The control groups for each of the four scenarios did not receive unloading treatments.
In the groups with a shorter duration of ischaemia, ischaemia alone did not generate any significant infarct scar; only under ischaemia followed by reperfusion (I/R) was a significant infarct scar produced, and this was significantly reduced by LV unloading. For the groups subjected to prolonged ischaemia, a small infarct zone was observed with ischaemia alone, whereas I/R presented a much larger infarct zone. In both cases, infarct size was significantly reduced by Impella unloading compared with control. Taken together, LV unloading initiated prior to reperfusion greatly limited infarct size in both short and prolonged I/R scenarios.
Dr Swain measured the activity of mitochondrial complex I, a critical enzyme in the respiratory chain, in hearts collected from all four groups ± unloading. Complex I activity was preserved in all four scenarios with transvalvular unloading. HIF-1α protein expression was also assessed; interestingly, HIF-1α expression declined with unloading under ischaemia-only conditions (Groups A and C), regardless of ischaemia duration. This suggests reduced ischaemic burden in the infarct zone of unloaded hearts and consequently destabilised HIF-1α proteins. Under I/R conditions (Groups B and D), HIF-1α protein levels increased with unloading in both groups. To understand whether the observed changes in HIF-1α protein levels are functionally relevant, Dr Swain measured the expression of HIF downstream targets prolyl-4-hydroxylase domain 3 (PHD3) and glucose transporter 1 (Glut1). The expression patterns of both proteins similarly matched that of HIF-1α. To demonstrate HIF-1α is driving the cardioprotective effect of unloading in I/R, Dr Swain infused 2-methoxyestradiol, a chemical inhibitor of HIF-1α, during transvalvular unloading in the prolonged I/R model (Group D). She confirmed that inhibitor infusion led to significant downregulation of HIF-1α protein in the heart. Inhibition of HIF-1α significantly attenuated the cardioprotective effects of unloading, as evidenced by increased infarct size and reduced complex I activity. This study suggested that HIF-1 is critical to the protective effects of unloading in I/R.
Dr Swain concluded that this work introduces HIF-1α as a major molecular player involved in cardioprotective effects of LV unloading in I/R.