Transcatheter aortic valve implantation (TAVI) has improved outcomes for many patients with aortic stenosis (AS), including high-risk and inoperable patients. However, some patients either have a high mortality despite TAVI or receive no symptomatic/functional benefit from the procedure. In the CoreValve US Pivotal Extreme and High Risk trials, TAVI was futile at 1 year in 50.8% of patients; 30.2% had died; quality of life (QoL) did not improve in 19.6%, and declined in 1.0%.1 Similarly, the PARTNER high-risk trial, showed that TAVI was futile in 40% of patients.2 Although technological, operator and pathway improvements have reduced mortality and complications since its inception, TAVI remains expensive, invasive and carries risk.3 TAVI studies have primarily focused on identifying predictors of mortality and major adverse cardiovascular events; however, many elderly TAVI patients value different treatment goals, such as independence and QoL.
Current guidelines define futility as a lack of survival or improvement in QoL/symptoms at 1 year post-TAVI and do not recommend intervention for AS if TAVI is deemed futile.4 Although predicting outcomes and making management decisions can be challenging, it is becoming increasingly important as the utility of TAVI expands.
This review aims to provide clarity on the topic by discussing the decisions regarding futility, evaluating the impact of comorbidities on both mortality and functional outcomes, and importantly, which comorbidities can improve following TAVI. Although not exhaustive, the comorbidities discussed here represent those that are relevant and most influential in a high-risk/inoperable population with severe symptomatic AS. Asymptomatic patients are not discussed in this review, but may benefit from TAVI, largely from reducing the risk of mortality from AS and associated comorbidities.
Methods
PubMed was searched for articles relating to TAVI in high-risk and prohibitive-risk patients with severe symptomatic AS between 2010 and 2020. The following free-text terms were used to identify relevant references: predictors of outcomes, mortality, functional outcomes, symptomatic changes and futility. Articles were screened for their relevance to the topic and excluded if they were not relevant, were duplicates or not in English. Additional references were identified by searching reference lists of included articles and guidelines. Comorbidities were then selected based on their relative impact on futility, consistency in the literature, and both their relevance and prevalence in the high-risk/surgically inoperable TAVI population. This was discussed and decided upon by all authors. The quality of each reference was checked by two authors (KPP and MJM). All authors were involved in providing expert opinion to interpret the data and formulate recommendations.
This review article discusses comorbidities associated with futility in TAVI and those that can improve with TAVI. It then focuses on patient evaluation, challenges in grading the severity of AS and symptom assessment. Finally, it brings together all these elements into validated risk stratification tools, discussing their merits and limitations, before describing the pivotal role played by the multidisciplinary team.
Cardiac Conditions Affecting TAVI Outcomes
AF
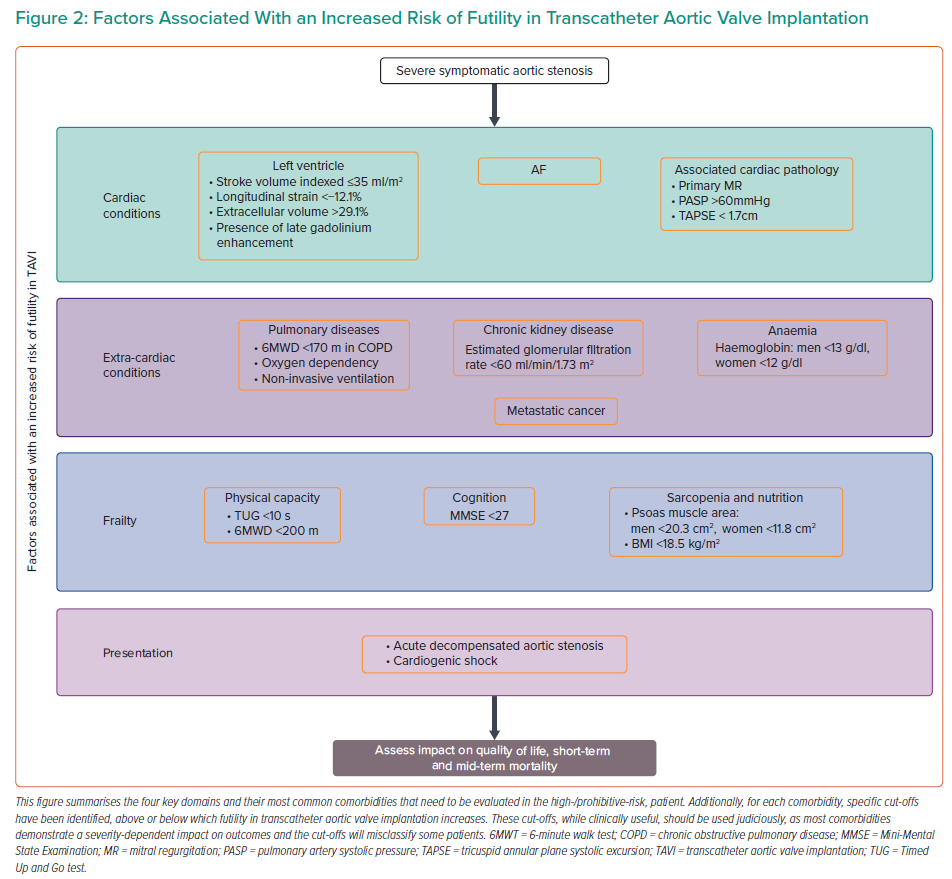
Although AF is a marker of increased morbidity, it has been shown to independently predict mortality at 1 year (HR compared with sinus rhythm 1.88–2.36), but not at 30 days.5–7 Mortality is often related to heart failure; however, renal failure, thromboembolic disease and mitral regurgitation are all associated with AF, and are likely to contribute to mortality.8,9 The risk increases with higher heart rate and CHA2DS2 VASc scores, supporting the importance of rate-control and comorbidities in determining futility.5,6 Stroke post-TAVI is an important determinant of functionality and quality of life. Pre-TAVI AF has not been shown to increase the risk of stroke, whereas new AF post-TAVI does.7 This is likely to be due to differences in antithrombotic treatment.10
Left Ventricular Function and Structure
Left ventricular systolic dysfunction independently increases mortality from heart failure and sudden cardiac death post-TAVI, with worse function conferring a higher risk.11 However, low transvalvular flow (measured as indexed stroke volume ≤35 ml/m2) may be a better prognostic marker than left ventricular ejection fraction (LVEF). This is supported by poorer outcomes in patients with paradoxical low-flow, low-gradient (LFLG) aortic stenosis (AS; where LVEF is normal) and a study where low flow remained an independent predictor of mortality (HR 1.29; 95% CI [1.03–1.62]), but LVEF and mean gradient did not.12 Thus, the effect of low forward flow might be more important than the mechanism causing it. It should be noted that despite poor outcomes compared with normal-flow, high-gradient patients, those with LFLG have a lower mortality with TAVI than with conservative treatment (HR 0.36; 95% CI [0.24–0.55]; p<0.001).13 This is the case for both classical LFLG AS (HR 0.43; 95% CI [0.19–0.98]; p=0.04) and paradoxical LFLG AS (HR 0.38; 95% CI [0.16–0.87]; p=0.02).14 Among survivors, functional outcomes at 1 year post-TAVI with low flow are comparable to normal flow patients.12
Left ventricular systolic dysfunction can also be reversible in AS patients, with improvements seen in up to two-thirds of patients as early as 48 hours post-TAVI and continued up to over 1 year post-TAVI. Determinants of improvement in left ventricular systolic dysfunction are high transvalvular gradient at baseline and the absence of a permanent pacemaker.15
Even among patients with preserved LVEF, further refinement of risk is beneficial. Strain imaging is a more sensitive marker of LV systolic function than LVEF. Studies have demonstrated among patients with preserved LVEF, longitudinal strain can predict mortality over and above traditional risk factors (for every 1% increase in longitudinal strain HR 1.05–1.42; p<0.0001).16,17 A marked impact on mortality was observed in patients with longitudinal strain <−12.1% compared with better strain values (10% had died at 1 year).17
Cardiac fibrosis, which can either be reversible interstitial fibrosis or irreversible replacement fibrosis, develops as part of the remodelling process in AS, and in the case of replacement fibrosis, can be associated with previous MI. Replacement fibrosis, particularly in the mid-wall, identified using late gadolinium enhancement on cardiac MRI, independently predicts mortality (HR 5.35; 95% CI [1.16–24.56]).18 It also precludes favourable reverse remodelling post-TAVI, but does not affect changes in LVEF.19 Extracellular volume measured using cardiac MRI, is a surrogate marker for diffuse fibrosis. Bearing in mind the constituents of extracellular space, one study demonstrated it independently predicts mortality after aortic valve replacement at a median of 3.8 years (HR per percentage increase in extracellular volume percentage: 1.10; 95% CI [1.02–1.19]). The study demonstrated 52.7 deaths per 1,000 patient years with an extracellular volume percentage >29.1%.20
Transthyretin amyloidosis (ATTR) has been identified as a common comorbidity in TAVI patients (13–16%).21,22 TAVI has been shown to improve outcomes among patients with coexisting AS and ATTR compared with medical therapy (p=0.03). Compared with patients with only AS, patients with AS and ATTR had a similar mortality (23% versus 21%; p=0.71) and procedural complications were similar (p=0.77).21 Prospective studies are required to ascertain functional outcomes and reverse remodelling in patients with coexisting AS and ATTR.
Left ventricular function, mitral regurgitation (MR), pulmonary hypertension (PH) and right ventricular dysfunction (RVD) are inextricably linked, such that each pathology influences the others. Therefore, teasing out the contribution of individual diseases to outcomes is challenging, creating controversy among studies.
Mitral Regurgitation, Pulmonary Hypertension and Right Ventricular Dysfunction
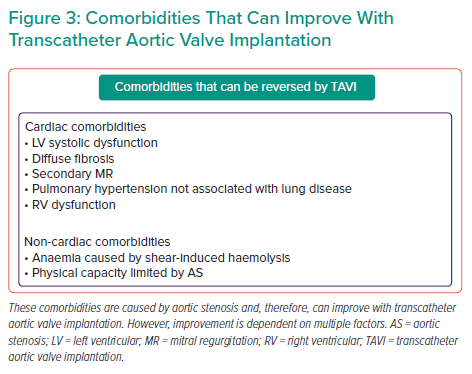
MR independently increases mortality at both 30 days (effect size −0.18; 95% [CI 0.31, −0.04]) and 1 year (effect size −0.22, 95% CI [−0.36, −0.08]).23 Despite this, TAVI in patients with ≥moderate MR remains better than medical therapy for improving mortality at 1 year (HR 0.38; 95% CI [0.019–0.75]).24 TAVI can also reduce MR; patients with functional MR, and the absence of severe pulmonary hypertension, AF and coronary artery disease increased the likelihood of such an improvement.25,26 Between 51% and 58% of patients with moderate/severe functional MR at baseline experience at least one or more grade improvement in MR at 1 year.26–28
Another observational study demonstrated moderate/severe MR improved in 79% of patients with functional aetiology compared with 50% of those with primary aetiology (p=0.025).25 Interestingly, among patients where ≥MR persists post-TAVI, left ventricular reverse remodelling, improvement in symptoms and New York Heat Association functional class do not seem to be adversely affected.29 This suggests that the risk of futility with TAVI increases with primary MR and the presence of associated comorbidities. With advances in transcatheter techniques, patients in whom TAVI does not reduce MR, transcatheter mitral valve repair/replacement can be an option; with initial studies demonstrating high procedural success, an acceptable safety profile and an improvement in symptoms.30,31 However, as we have learned from the COAPT and MITRA-FR studies, patient selection is key to achieving benefit.32
MR pre- or post-TAVI increases left atrial volume and pressure that eventually can result in PH. A meta-analysis of TAVI patients demonstrated that PH (defined as pulmonary artery systolic pressure >60 mmHg) increased the risk of all-cause mortality both at 30 days (OR 1.48; 95% CI [1.17–1.88]) and at 1 year (OR 1.39; 95% CI [1.24–1.57]), along with acute kidney injury at 30 days and stroke at 1 year.33 Pre-capillary and combined PH, and increasing severity of PH confer a higher risk of mortality.34,35 Persistence of PH, regardless of its aetiology, seems to be more important than baseline PH in predicting outcomes. Approximately half of patients with PH have immediate improvement in pulmonary artery systolic pressure post-TAVI, which is sustained up to a year.36 This improvement is more likely with a LVEF >40%, functional MR, mild diastolic dysfunction and in the absence of moderate to severe TR and AF.36,37
By comparison, PH caused by chronic lung disease or associated with pulmonary vascular remodelling is less likely to improve with TAVI.34 Functional class improves regardless of baseline PH, again suggesting that if AS is the dominant pathology, patients are likely to benefit from TAVI.38 Therefore, among patients with PH, the risk of futility increases with the severity of PH, associated comorbidities and non-AS related aetiology of PH.
RVD is often the consequence of transmitted pressure from the AS-loaded left ventricle, but co-existing pulmonary disease and other causes of precapillary PH do contribute. RVD (defined as tricuspid annular plane systolic excursion <1.7 cm) is prognostically important (HR at 12 and 43 months for all-cause mortality 2.94; 95% CI [2.02–4.27] and 2.14; 95% CI [1.31–3.51]; p<0.001, respectively).39,40 Over half of patients with baseline RVD demonstrate RV functional recovery within days post-TAVI, which is likely to be due to the reduction in LV afterload. Among patients in whom RVD did not recover, mortality (particularly early mortality) is up to eightfold higher. AF and a lower LVEF reduce the chances of recovery.39 Further work is required to determine the extent of symptomatic benefit that patients with RVD derive.
Extra-cardiac Conditions Affecting TAVI Outcomes
Anaemia
Anaemia is associated with a poorer prognosis in a severity-dependant manner and affects mortality at 1 year (haemoglobin <10 g/dl, HR 2.78; 95% CI [1.60–4.82]; haemoglobin <13 g/dl for men and <12 g/dl for women, HR 2.10; 95% C: [1.06–4.18]) rather than at 30 days, and increases rates of hospitalisation due to heart failure.41–44 However, TAVI can also lead to the resolution of pre-existing anaemia; particularly that caused by AS-induced intravascular haemolysis and Von Willebrand factor cleavage.45 Post-TAVI anaemia rather than baseline anaemia is predictive of a poor symptomatic response to TAVI, indicating that non-AS-related causes of anaemia (such as renal failure) that persist post-TAVI are likely to affect outcomes, including symptom improvement.46 Targeting a treatable cause of anaemia in AS patients; for example with iron therapy, needs to be evaluated in prospective studies.
Chronic Lung Disease
Both chronic obstructive pulmonary disease (COPD) and restrictive lung disease increase the risk of mortality, in the short and long term (HR for 1-year all-cause mortality for COPD 1.09–1.46, 95% CI [1.02–1.79]; HR for restrictive lung disease 2.25, 95% CI [1.35–3.75]).47,48 Poor exercise tolerance measured using a 6-minute walk test (6MWT), oxygen dependency and the use of non-invasive ventilation are recognised predictors of TAVI futility.48,49 These factors all indicate a higher severity of lung disease. Consequently, patients with CLD stand to gain less of an improvement in New York Heat Association status with TAVI, although up to 80% of them can experience some improvement.49,50 The rate of futility among TAVI patients with CLD can be as high as 57% at 1 year. In addition to the predictors of futility mentioned above, lower diffusing capacity of the lung for carbon monoxide has been identified as a pulmonary-specific predictor of futility.50
Chronic Kidney Disease
CKD is a predictor of both 30-day and 1-year mortality in a severity-dependent manner (every 10-ml/min/1.73 m2 reduction in baseline estimated glomerular filtration rate increases mortality by 4.4%).51 Patients on dialysis have an approximately twofold increase in mortality compared with non-dialysis patients.52 It also increases the risk of bleeding and stroke among higher-risk patients.53 Despite this increased risk, TAVI is a better option than medical treatment, with lower mortality rates (at a mean of 1.9 years, HR of mortality with medical management compared with TAVI 3.95; 95% CI [2.59–6.02]) and potential stabilisation of renal function.54 CKD has been identified as an independent predictor of lack of improvement in functional status, in a severity-dependent manner, mainly due to associated comorbidities, such as anaemia and sarcopenia.46
Malignancy
This heterogenous group of pathologies with varying prognosis based on type, extent and treatment is common in the elderly – one study revealed a 5.4% prevalence of active cancer and 13.8% of a prior history of cancer among TAVI patients.55 The majority of cancers are prognostically important, and among TAVI patients have been shown to account for 7% of deaths at 30 days, and between 2 and 8.6% of deaths at 1 year.3,56,57 There is heterogeneity in the literature regarding outcomes in patients with cancer. At 1 year post-TAVI, mortality was higher (37.4 versus 20.8%; p<0.001) and improvement in functional class was lower among patients with active cancer compared with those without cancer.55 Another study demonstrated that active cancer does not affect TAVI procedural success and complications, and that at a median on 272 days, mortality was similar between the cancer and non-cancer group (p=0.42). However, the presence of metastatic cancer was an independent predictor of mortality (HR 4.73, 95% CI [1.12-29.0]; p=0.035).58 However, selection bias and confounding factors, such as anaemia and sarcopenia, which tend to coexist with cancer, were not accounted for in both studies. Incidental masses among elderly patients can be found in one in five patients who undergo pre-TAVI CT, with solitary lung nodules being the most common finding. As an entity, incidental masses do not affect outcomes, and the majority are benign. However, among patients with a prior history of cancer where it is more likely to represent malignancy, incidental masses are associated with increased 1-year mortality (OR 4.02; 95% CI [1.5–10.7]; p=0.006).59 Incidental masses may result in further investigations for a patient, providing an opportunity for commencing treatment if appropriate.
For patients in this complex disease group, a tailored approach for each individual is required; with consideration of whether TAVI can facilitate further oncological treatments, such as surgery, and an evaluation by a multidisciplinary team involving an oncologist.
Frailty and Related Conditions Affecting TAVI Outcomes
Frailty is a state of decreased functional and physiological reserve, and is often caused by the accumulation of health deficits. Understanding frailty helps predict outcomes, stratify risk, and identify patient-specific targets and outcomes. It can also identify patients who may benefit from frailty-specific interventions. Trials assessing the effectiveness of interventions on frailty among TAVI patients are still awaited; however, these interventions have proved to be beneficial in other populations (NCT03107897 and NCT0352245). Physical, nutritional, and cognitive interventions can improve frailty scores and status at 12 months.60 Intensive exercise leads to greater improvements in disability and physical functioning compared with light exercise.61 Nutritional supplementation in older patients demonstrated an improvement in quality of life and physical functioning.62
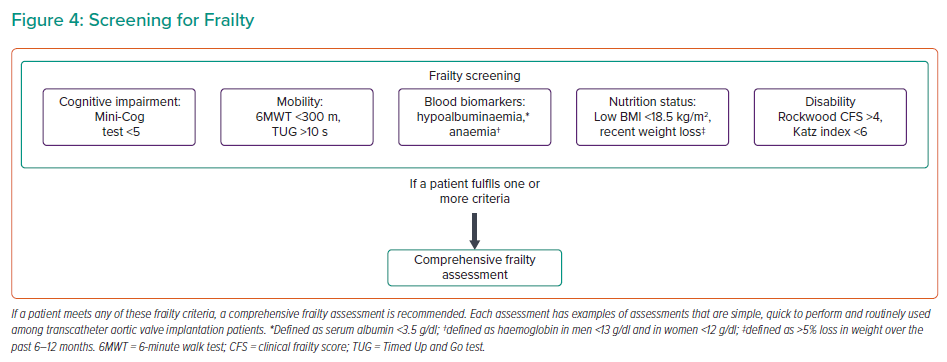
The assessment of frailty has generated enormous interest within the TAVI community, with the development of several scoring systems. Consequently, the reported prevalence of frailty varies between 6% and 90%.63,64 Determining which score to use, balancing a comprehensive frailty assessment with a busy clinical workload and determining what to do once frailty has been recognised are challenging. Below, we provide a summary of the main domains of frailty with validated thresholds for futility. Regardless of how frailty is assessed, it is associated with a poor prognosis. Two meta-analyses demonstrated that frailty is an independent predictor of mortality at ≤30 days (HR 2.35; 95% CI [1.78–3.09]), >30 day (HR 1.63; 95% CI [1.34–1.97]) and at 1 year (HR 2.16, 95% CI [1.57–3.00]).65,66 Frailty also predicts functional decline post-TAVI (OR 1.82; 95% CI [1.14–2.91]).67
Physical Capacity
Mobility is a significant contributor to frailty and is often used to approximate its presence. Patients with AS and low physical capacity, determined by either a 6MWT and Timed Up and Go test have a poorer prognosis than those with higher capacities.49,68 A 6MWT <170 m was identified as the optimum cut-off to predict futility at 6 months among patients with COPD undergoing TAVI (area under the receiver operating characteristic curve 0.67).49 One study showed that for every 10 m walked during a 6MWT, the risk of a poor outcome reduced by 3%.69
Patients with Timed Up and Go test times between 10 and 20 seconds have a greater than fivefold increase in mortality at 1 year, compared with patients with a Timed Up and Go test time <10 seconds.68 However, defining specific cut-off points to determine futility using any of these continuous variables is clinically useful, but will misclassify some patients and, therefore, should be used judiciously. Additionally, if a patient’s mobility is limited by AS, those with lower values stand to gain the most functional benefit from TAVI.70 The key to determining futility is to identify physical limitations caused by non-AS related pathologies, which will not improve with TAVI.
Cognitive Impairment
Cognitive impairment is under-recognised and a significant contributor to frailty.71 Patients with cognitive impairment at baseline (Mini Mental State Examination score <27) have more than a threefold increased risk of functional decline or mortality at 1-year post-TAVI.72 Another study showed that for every point gained on the Mini-Mental Test score, the OR of a poor outcome was 0.94 (95% CI [0.90–0.97]; p=0.001).71
Sarcopenia and Nutrition
Sarcopenia is a state of low muscle mass, strength and function, and is present in one-third of elderly patients.73 Psoas muscle area and volume act as surrogate markers of sarcopenia, and are calculated using pre-TAVI CT scans. Sarcopenia (psoas muscle area: men <20.3 cm2 and women <11.8 cm2) has been shown to predict mortality and worsening disability at 1 year.74,75 Up to 42% of patients undergoing TAVI are either at risk of malnourishment or are malnourished. These patients have more comorbidities and a lower BMI.76 Lower BMI (<18.5 kg/m2) at baseline is associated with increased mortality at 1 year rather than at 30 days, whereas, paradoxically, obese and overweight patients tend to have a survival advantage at 1 year.77 Functional outcomes among malnourished TAVI patients are unknown.
Outcomes in Specific Populations
Acute Decompensated Aortic Stenosis
Acute decompensated AS (ADAS) is defined by debilitating symptoms related to AS (syncope, angina with minimal exertion or at rest and/or dyspnoea at rest). The condition frequently warrants hospitalisation and urgent valve replacement. Although TAVI has been performed safely in these patients, outcomes are worse than patients without decompensation; at 1 year post-TAVI, mortality is between 15.3 and 29.1%.78–80 Traditional markers of futility described above predict mortality in ADAS: AF, oxygen-dependent lung disease, low body surface area (a marker of sarcopenia/malnutrition), previous cardiac surgery and poor renal function.80 However, there is a large degree of overlap in baseline characteristics between ADAS and non-ADAS patients, making it challenging to differentiate and, therefore, predict futility.
Among patients presenting with acute decompensation is a subgroup with cardiogenic shock. Data on TAVI within this subgroup are limited to small case series. Device success is reportedly high (94%), However, Valve Academic Research Consortium-2-defined early safety endpoints were reached in 35% of patients, with 30-day mortality of 12–24%.81,82 At 1 year, mortality was reported at 26% and related to non-cardiovascular causes in the majority of patients. However, among survivors, TAVI did improve symptoms; 91% were New York Heat Association class I or class II.81 For patients with ADAS, non-randomised data suggest that TAVI is a better therapeutic option than balloon aortic valvuloplasty.80,82
Patient Evaluation
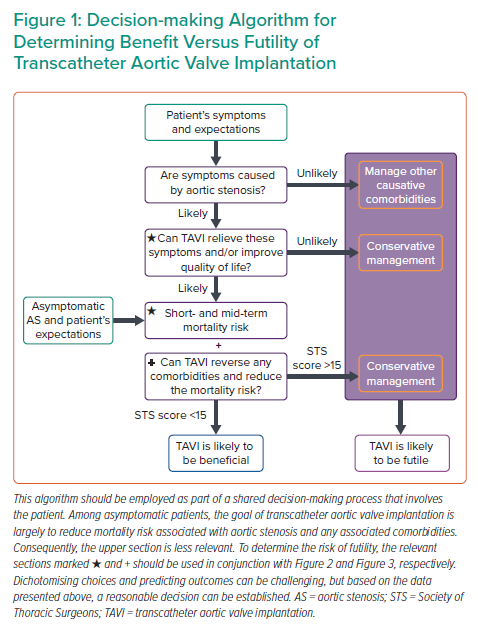
By following a systematic approach, as suggested in Figure 1, using available evidence where present and clinical judgment where absent, a reasonable management decision can be made. Once the severity of AS is established, the next step involves symptom assessment to establish causality and explore a patient’s expectations. The third step involves evaluation of a patient’s comorbidities, their impact on mortality, symptoms, and quality of life with and without TAVI. Figure 2 identifies specific cut-offs for factors within four key domains that are associated with futility in TAVI. Many factors affect outcomes in a severity-dependent manner, therefore, while specific cut-offs are clinically useful, some patients will be misclassified and, therefore, should be used judiciously. The final step that lends support to the decision-making process is whether TAVI can reverse existing comorbidities to improve outcomes. Figure 3 identifies comorbidities that tend to improve with TAVI. However, improvement in each is dependent on several other factors discussed above. Shared decision-making within a multidisciplinary team and with the patient is a pivotal part of this entire process.
Evaluation of Aortic Stenosis
Defining the severity of AS is important to justify the risk–benefit balance associated with TAVI; the higher the severity of AS, the greater the benefit of TAVI.
Severe AS is straightforward to define when echocardiographic markers are concordant (peak velocity ≥4m/s, mean gradient ≥40 mmHg and aortic valve area ≤1 cm2). However, these markers can often be discordant if transvalvular flow is reduced (≤35 ml/m2). A detailed review of diagnostic challenges and solutions for low-gradient AS can be found elsewhere.83,84 However, two investigations are worth mentioning here. To differentiate between severe AS and pseudo-severe AS, low-dose dobutamine stress echocardiography can be helpful. By iatrogenically increasing the flow to >35 ml/m2, valve haemodynamics can be recalculated at normal flow. If, however, flow cannot be increased sufficiently, the projected aortic valve area can be calculated, as described by Blais et al.85 Dobutamine stress echocardiography can also provide a measure of contractile reserve (increase in stroke volume by 20%). While the presence of contractile reserve is prognostically important in patients undergoing surgical aortic valve replacement, it does not influence outcomes among TAVI patients.86,87 Furthermore, the aortic valve calcium score using CT can be beneficial to identify severe AS with established sex-specific cut-offs.88 Among elderly, high-risk patients, this is a useful tool; however, in younger patients with bicuspid AS, valve calcification plays less of a role in the pathobiology of AS, and the CT valve calcium score may underestimate the severity of AS.89
Symptom Evaluation
Identifying a patient’s symptoms and assessing the contribution made by AS is key. Multimorbidity makes attributing symptoms to a particular disease challenging; for example, distinguishing dyspnoea from severe AS versus COPD. If dyspnoea worsens with a simultaneous increase in AS severity and little change in lung function, it is likely that AS is the driving cause. Appreciating a patient’s expectations and whether these can be met with TAVI is important. Using the example above, even with successful TAVI, COPD cannot be cured, and a degree of dyspnoea is likely to remain. It is important that the patient understands this.
Patients who have the least to gain from TAVI in terms of symptom benefit and improvements in QoL are those with mild or no symptoms, alternative causes contributing to their symptoms and phenotypic changes (e.g. certain features of frailty) that cannot be reversed with TAVI.
Risk Assessment for High-risk Patients
Scoring Systems
Several frailty parameters and risk scores can be inaccurate, exclude important facets of frailty or require extensive assessments, discouraging their use in the clinical arena.72,90,91 Therefore, we propose a simple screening tool to identify frail patients (Figure 4) who would benefit from a more thorough assessment, preferably by a geriatrician.60,62 The cut-offs chosen for each domain have demonstrated prognostic or diagnostic importance.68,92–99 Included in this screening tool are several factors discussed above. In addition, assessing disability and independence using validated tools, such as the Rockwood Clinical Frailty Score and the Katz index, although semi-quantitative tools, can provide quick and important prognostic data for TAVI patients.66,99,100 Interventions to improve frailty and their impact on outcomes are ongoing, limiting the role of comprehensive frailty assessment to risk stratification rather than therapeutic interventions (NCT03107897 and NCT0352245).101,102
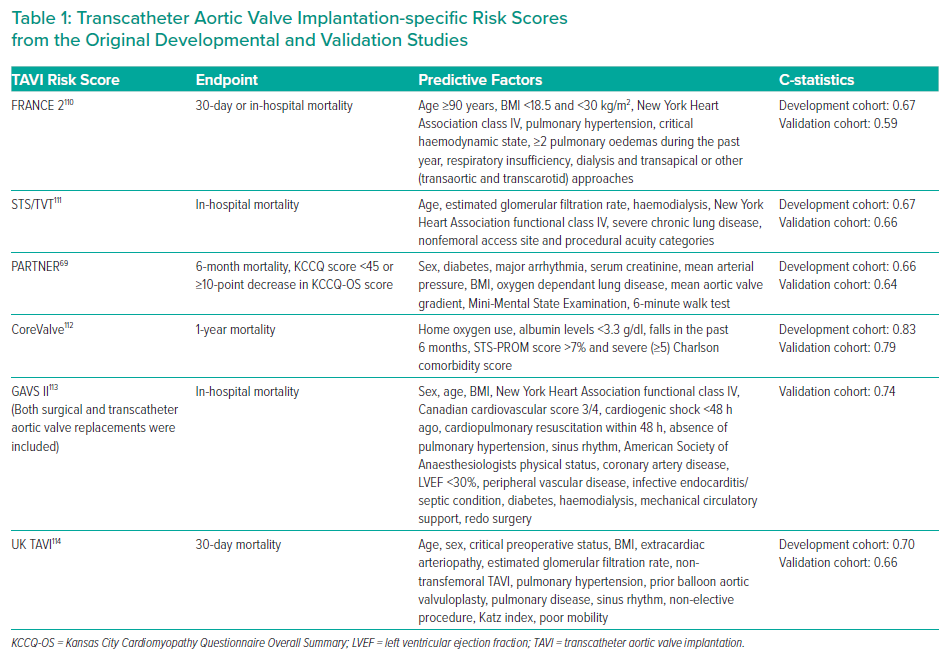
Although risk stratification using TAVI-specific tools provides a similar or better estimation of mortality compared with traditional surgical risk scores, further refinement is required.103,104 Table 1 summarises the predictors used in several TAVI-specific risk scores and their corresponding C-statistics. The Society of Thoracic Surgeons (STS)/American College of Cardiology Transcatheter Valve Therapy TAVI score demonstrated an area under the receiver operating characteristic curve of 0.64 in determining 30-day mortality with better discrimination of mortality compared with STS-Predicted Risk of Mortality score for high-risk patients.105 Compared with surgical risk scores (Euroscore 2 and STS), the French Aortic National CoreValve and Edwards 2 (FRANCE 2) score had a lower, albeit non-significant C-statistic (0.67 versus 0.53; p=0.26).106
However, most scores do not predict functional or symptomatic improvements, which for many patients are equally if not more important. Newer tools are taking into consideration both functional/symptomatic outcomes and mortality. One model, predicting the composite of a poor QoL, decrease in QoL and mortality at 1 year, demonstrated moderate discriminatory ability (C-statistic 0.66). Using this model, among patients in a validation cohort judged to be at very high risk (>70% risk of the composite endpoint), 73% met the composite endpoint – demonstrating good predictive ability among this subpopulation.71
The Essential Frailty Toolset has been shown to be one of the strongest predictors of mortality at 30 days (OR 3.29; 95% CI [1.73–6.26]) and 1 year (OR 3.72; 95% CI [2.54–5.45]), as well as worsening disability at 1 year (OR 2.13; 95% CI [1.57–2.87]), compared with other scoring systems.90 The addition of the Essential Frailty Toolset to STS-PROM shows promise with a C-statistic of 0.83. If classified as severely frail using the Essential Frailty Toolset (5/5), patients had a 80% risk of mortality or disability at 1 year.90,107 The combination of these two scoring systems could prove to be a reliable tool to determine futility, and requires prospective studies to validate it.
Decisions based on any scoring system need to be made around a patient-centred approach. Patients at very high-risk (STS >15%) do not have a survival benefit compared with conservative treatment.108 These newer risk stratification tools need to be validated in different populations, and will need to constantly evolve as TAVI evolves and novel predictors of futility are identified. Future studies are required to address whether frailty can be improved by treating particular factors, such as malnutrition, and whether this can improve TAVI outcomes.
Role of the Multidisciplinary Team
The multidisciplinary team has become the cornerstone for making complex management recommendations and is advocated by international guidelines.91,109 It is particularly helpful, where equipoise exists regarding the benefit/futility of TAVI. In the multimorbid, frail patient, a geriatrician is invaluable to guide this process. If TAVI is not recommended by the multidisciplinary team, it is important to sensitively convey this to the patient and their relatives. A discussion should be had regarding the patient’s thoughts, concerns and expectations, and the rationale for the recommendation. This discussion can then form the basis of the final decision. If a clinical decision has been made to avoid TAVI because of probable futility, palliative care should be involved to alleviate symptoms, personalise care, provide psychological support and ensure good lines of communication for the patient.
Conclusion
The more comorbidities a patient has, the lower the chances of an improvement in physical and psychological quality of life, and the higher the mortality rate. Additionally, the severity of these comorbidities is important, with higher severity pertaining a higher risk of futility. Futility should be considered, especially in patients whose health is affected primarily by comorbidities other than AS. It is important to consider certain comorbidities that can reverse post-TAVI (e.g. functional MR), despite conferring excess risk. Quantifying the contribution of specific comorbidities to a patient’s symptoms can facilitate better prediction of symptomatic improvement and allow patient expectations from TAVI to be managed. Therefore, both patients and clinicians need to be clear about the potential improvements that TAVI can provide.
Although our understanding of comorbidities and their impact on TAVI outcomes has improved, there is still a need to refine our prediction tools, and better understand the impact of TAVI on QoL and function, such that this rapidly growing technology is targeted towards those patients who are likely to gain the most benefit and avoided amongst those where it will be futile.
Clinical Perspective
- Futility in transcatheter aortic valve implantation (TAVI) is common and should be avoided. Up to half of high-risk patients undergoing TAVI do not gain any improvement in quality of life (QoL), symptoms or survival at 1 year.
- The number and severity of comorbidities increase the risk of futility.
- However, certain comorbidities can be reversed with TAVI, thus improving outcomes; for example, functional mitral regurgitation and anaemia caused by intravascular haemolysis.
- Screening and a comprehensive assessment of comorbidities and frailty can lead to better risk prediction and reduce futility.
- Further research is required to identify predictors of a lack of improvement in QoL and function.