Dr Faraz discussed the ‘Hack Attack’ program for the ST-Segment Elevation MI Door-to-Unloading (STEMI-DTU) pivotal trial, covering the process of identifying and enrolling eligible patients at any time of day, any day of the week (24/7). The STEMI-DTU pivotal trial is a prospective multicentre double-arm randomised control trial evaluating patients undergoing Impella or standard of care treatment for a STEMI heart attack.1 Despite Hackensack University Medical Center being a large-volume tertiary centre with an annual STEMI volume of >100, the initial enrolment for the STEMI-DTU pivotal trial was low, with only 8 of 107 STEMI patients (7.5%) screened for the trial, resulting in one enrolment in 2020. Similarly, in 2021, 22 of 110 STEMI patients (20%) were screened, with only six being enrolled in the trial. Dr Faraz recognised that in order to successfully translate scientific findings to clinical application through a clinical trial, maximising the screening of potential study candidates is crucial because a significant portion of candidates get filtered out by the eligibility criteria.
In order for the clinical trial to enrol enough patients to be successful, the principal investigator (PI) at each site must actively mobilise system resources to maximise possible enrolment. The PI is the primary person responsible for evaluating patients for enrolment in the STEMI-DTU trial; at Hackensack University Medical Center, the PI has a team working together to manage 24/7 enrolment. This team includes four interventionalist faculty members, who can serve in place of the PI in case of multiple simultaneous STEMI presentations, and provide consultations to non-faculty staff for trial screening and enrolment. In addition, eight practicing interventionalists provide 24/7 primary percutaneous coronary intervention (PCI) coverage for STEMI, and these interventionalists are educated and encouraged to notify faculty about potential STEMI-DTU trial candidates.
Dr Faraz noted that due to the 24/7 nature of the enrolment process, the success of the STEMI-DTU trial relies on everyone in the team playing their part. First, the emergency department (ED) physicians have to recognise and activate STEMI in a timely fashion; the on-call cardiologists follow by properly recognising the anterior STEMI without prior cardiac arrest or shock as a potential DTU patient and consult the faculty involved in the trial. The faculty then screens and subsequently qualifies the patient for enrolment after discussion with the national committee via TigerConnect. Research nurses and administrative staff are responsible for data collection and submission. As an example, Dr Faraz provided a case review of a DTU randomised patient, sharing the timeline from STEMI activation through to 6-month follow-up. The patient was activated on a Sunday afternoon in the ED; within 20 minutes, the patient was qualified by the faculty and the national committee, and consented in the ED. Ten minutes after consenting, the patient was on the cath lab table; once femoral access and imaging had been obtained, the patient was randomised. By 1 hour after STEMI activation, the Impella was implanted, with a total DTU time of 81 minutes. Strong communication and teamwork were vital to the successful identification and enrolment of patients in the STEMI-DTU study, as events unfold quickly and require rapid assessment and decisions.
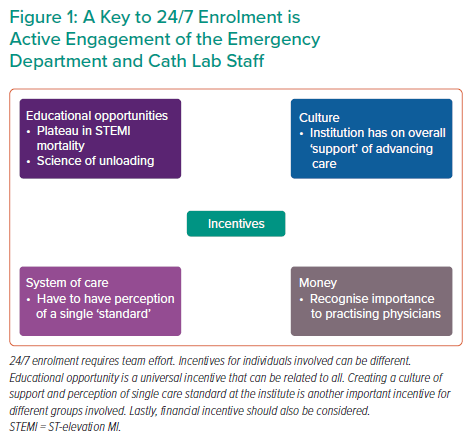
To achieve the highest success, there must be active engagement and incentives for all levels of the team (Figure 1). Dr Faraz concluded by noting that COVID-19 continues to be a significant obstacle to the STEMI-DTU study. He also emphasised that individual clinicians can be unaccepting of paradigm-shifting changes, uninvested in research and possess an intrinsic belief that they already know how to best treat patients presenting with STEMI. These intrinsic biases are significant obstacles to this type of trial and should be kept in mind when enrolling patients in the STEMI-DTU or other similar trials.









