The use of physiology to guide revascularisation in patients with coronary disease has been demonstrated to improve clinical outcomes and reduce costs.1,2 Despite this, its adoption into clinical practice is very low,3 moreover, when it is employed it is used simplistically – only to determine if the vessel is ischaemic or not.
Recently, several new indices of stenosis severity have been introduced that aim to improve adoption by addressing some of the major limitations of the existing clinical standard – fractional flow reserve (FFR).4,5 The most validated of these indices is the instantaneous wave-free ratio (iFR).4 This review will highlight the clinical problem in terms of hyperaemia-based indices, the physiological background of iFR, overview its validation studies and discuss the potential of ongoing clinical outcome studies.
Finally, the unique potential of hyperaemia-free indices will be discussed; to determine if they can fundamentally transform the use of physiology from a tool that can only ascertain
if revascularisation is required into a tool that can also guide how the revascularisation procedure should be performed.
The Clinical Problem
The main physiological foundation of FFR is that it can only make inferences about the haemodynamic significance of a stenosis under conditions of maximal hyperaemia.6,7 Superficial interpretation of this presumption has led some to believe that the haemodynamic significance of a stenosis can only be assessed during maximal hyperaemia.8–10
This has significant clinical and physiological implications that have directly impacted the adoption of FFR and simultaneously hindered the development of novel indices.7
Clinically, hyperaemic agents such as adenosine cannot be administered to all patients, almost always cause patient discomfort and their administration adds significant time to the procedure. Even in the most practiced hands, in institutions that routinely perform physiological measurements, an FFR assessment has been shown to add at least 10 minutes to the diagnostic procedure.11
Scientifically, the overly simplistic assumption that a single dose of adenosine can achieve maximal hyperaemia in every single patient regardless of myocardial territory and co-morbidity is now coming under increasing scientific scrutiny. A plethora of factors can influence the degree of hyperaemia achievable, including the dose of hyperaemic agent, its route of administration, left ventricular end diastolic pressure, right atrial pressure, age, gender, concomitant medication, diet and even the sleep pattern of the patient.12–20
Physiologically, it is increasingly evident that maximal hyperaemia is impossible to achieve in the catheter lab and given the sheer number
of variables affecting hyperaemia isolating the patients in which hyperaemia has been insufficient is equally impossible.21–23
This is particularly true of pressure-derived hyperaemic indices, such as FFR, which by definition do not measure flow and are therefore most vulnerable to this limitation.
This uncertainty has led clinicians to administer ever-increasing doses of adenosine and even adding other drugs to try and reassure themselves that ‘maximal hyperaemia’ is achieved. However, this only serves to create confusion because the FFR treatment threshold of 0.8 had not been validated against ischaemia at higher doses of adenosine making interpretation of the FFR value challenging and importantly not supported by outcome data. All these factors contribute to the poor ‘usability’ of FFR in the clinical domain – reflected in single figure adoption rates for FFR across most of the world despite a wealth of evidence being established for over 20 years.3
Can the Lessons of the Past Help us Find a Solution?
In recognition of the above limitations of FFR, hyperaemia-free indices have been introduced.4,5 These indices aim to circumvent the myriad limitations created by the need for administrating a hyperaemic agent in the hope of facilitated adoption by making procedures quicker, cheaper, safer and independent of the variability of attaining maximal hyperaemia. The most validated hyperaemia free index is iFR.4
iFR is not dependent on maximal hyperaemia and therefore shifts the paradigm away from maximal hyperaemia for the assessment of stenosis severity. This is based on a wealth of physiological data over the last 30 years that has clearly demonstrated that maximal hyperaemia is not required if stenosis assessment is made during a specific phase of the cardiac cycle.24–26
In a seminal experiment by Gould in 1978 it was demonstrated that the haemodynamic significance of stenoses could in fact be determined under basal conditions.24 Using combined pressure and flow velocity analysis he identified a phase in the cardiac cycle during which stenoses could reliably be differentiated into mild, moderate and severe. Using meticulous manual post hoc analysis of pressure and flow data he clearly demonstrated that the optimal period of the cardiac cycle to assess a stenosis was when intra-coronary haemodynamics are free of the confounding effects of the contracting and relaxing myocardium. This period within diastole has been studied in detail in several studies over the last 30 years and all indices derived over this period have been demonstrated to be at least as accurate as FFR and most importantly not reliant on maximal hyperaemia.26,27 Despite the clear physiological advantages of these indices over FFR, their clinical use was hindered by the need for simultaneous flow velocity measurements (which are challenging and time consuming to perform) and post hoc manual analysis of the pressure and flow traces; preventing real-time stenosis assessment.
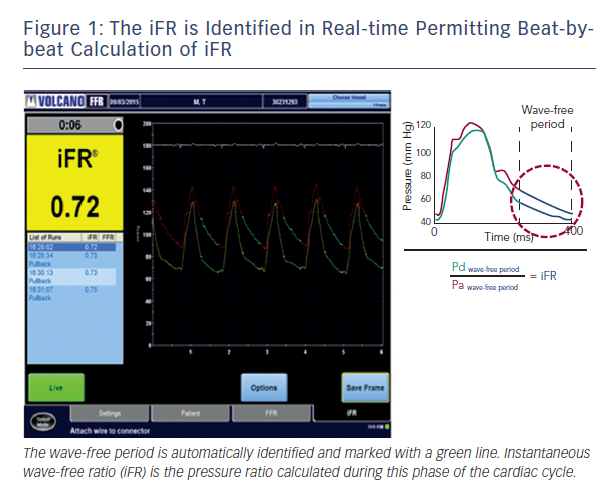
Recently, using combined pressure and flow assessment in humans wave intensity analysis identified a period within diastole when intra-coronary haemodynamics are also free of all wave activity, which is caused by relaxation and contraction of the myocardium.4 More importantly, using sophisticated computational algorithms this period can now be isolated in real-time on a beat-to-beat basis using the pressure waveform alone; making clinical use of this period feasible in daily clinical practice. It is during this period that iFR is calculated4 (see Figure 1).
Given the well-documented physiology of this period of the cardiac cycle and its unique suitability for stenosis assessment it is not surprising that iFR has been found to be able to determine the haemodynamic significance of stenoses. Furthermore because iFR can be measured within seconds, does not require hyperaemic agents and uses existing pressure wire technology its potential to improve adoption of physiology to realise the clinical and economic benefits of physiologically guided revascularisation is clear.28
iFR Validation Studies to Date
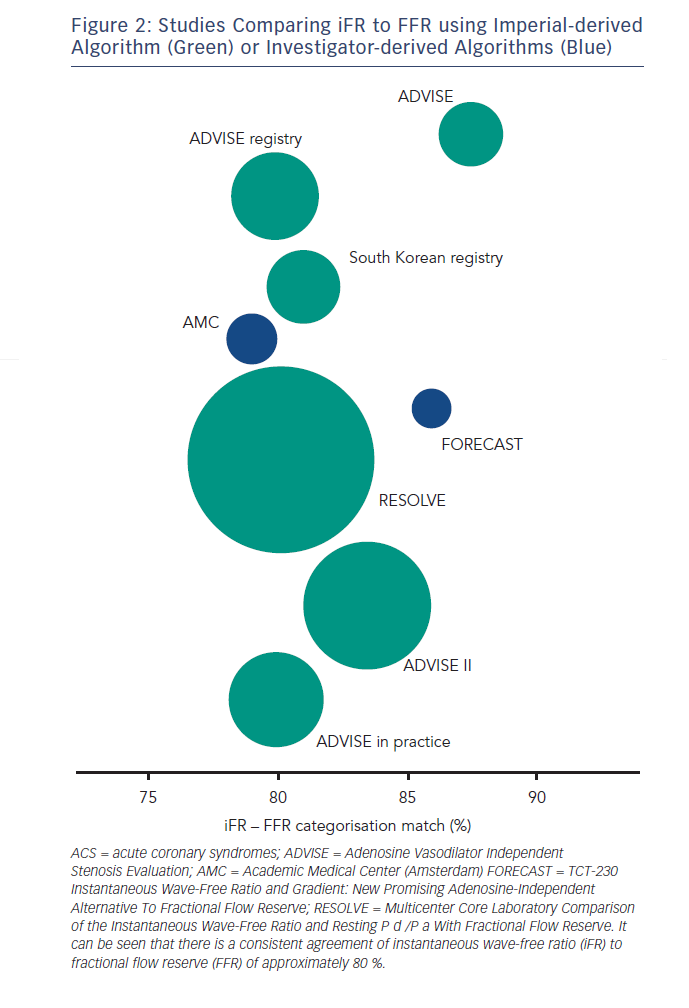
The classification match of iFR to FFR has been studied in over 3,500 vessels, this series of studies has definitively identified a treatment threshold of <0.9 for iFR.4,29–36
The agreement of iFR to FFR in the above studies has consistently been found to be approximately 80 % depending on the distribution of stenoses in the study population (see Figure 2). Interpretation of this statistic should take account of the fact that the classification match of two FFR measurements across the same lesion is not 100 % but 85 %.37,38 A finding suggested by the DEFER study39,40 but recently confirmed using modern pressure wire technology.41
Such classification agreement between two FFR measurements across the same lesion is not surprising given the biological variability of any physiological measurement especially if that measurement
is reliant on the heterogeneous and unpredictable effects of a hyperaemic agent.42
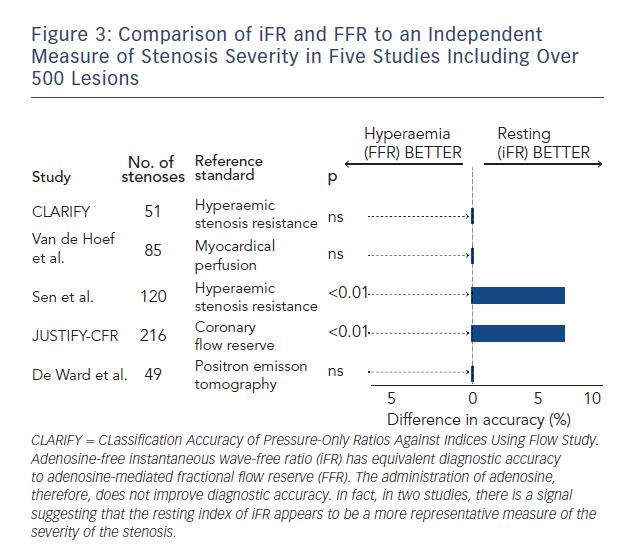
While FFR is viewed as a clinical standard for ischaemia it is widely accepted that there is no true gold standard test for ischaemia.43 Therefore, when two indices such as iFR and FFR agree so closely how do we interpret the significance of stenoses when iFR and FFR disagree? Several studies have attempted to look at this (see Figure 3).23,36,42–45 Each has taken iFR and FFR and compared with another index of stenosis severity. The salient findings of this body of research in over 500 stenoses are clear:
- When iFR and FFR disagree – FFR is not necessarily correct.
- Hyperaemia does not improve diagnostic accuracy.
The Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity-Coronary Flow Reserve (JUSTIFY-CFR) was the largest of these studies to systematically assess how iFR and FFR agree with underlying flow. It demonstrated that when iFR and FFR disagree that iFR was
more indicative of the underlying flow characteristics of the vessel.44 Furthermore in the clinically relevant 0.6–0.9 FFR range, in which most of our patients fall, iFR was significantly more accurate than FFR.
Some have highlighted concerns with regards to the doses of hyperaemic agent, choice of reference test and therefore the validity of these studies. The meticulous validation of FFR during its development serves as template for the validation of new indices. It should be noted that FFR was validated against the same reference tests during its development,
using doses of adenosine and routes of administration identical to the studies now comparing iFR with FFR. Should we discount these pivotal FFR studies too? If so, what should be done with the guidelines and scientific recommendations supporting FFR that were based upon these?
The above studies clearly challenge the dogma that hyperaemia is necessary for stenosis assessment but also point to major potential differences between iFR and FFR that may make iFR more clinically useful than FFR.
Indeed the ability of iFR to be independent from the response of the microcirculation to a hyperaemic agent suggests that, it may also have a role in conditions that have traditionally been considered unsuitable for FFR assessment. The assessment of disease in the acute coronary syndrome population, vessels with tandem lesions, patients with renal failure, diabetes and hypertension are all vulnerable to a variable response of the microcirculation to hyperaemic stimuli – a limitation circumvented with resting indices. The role of iFR and FFR in these populations will be further elucidated in the Functional Lesion Assessment of Intermediate Stenoses to guide Revascularisation (DEFINE-FLAIR) and iFR SWEDEHEART studies.
Randomised Clinical Outcome Studies Designed to Address the Clinically Important Questions
In reality the adoption of any new index will be based on large randomised clinical trials with hard outcome end-points. But for clinicians to be able to translate the results of these studies to the patients they treat the trials must include patients in which physiological assessment is routinely made in clinical practice.
Prior to the Adenosine Vasodilator Independent Stenosis Evaluation (ADVISE) registry FFR had never been systematically studied in clinically relevant patient populations.44 The mean FFR values in the Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention (FAME) and FAME II studies were 0.71 and 0.64, respectively; significantly lower than mean FFR values in contemporary studies that have included intermediate lesions.24,44 This suggests that the FAME populations are significantly different from the patients we assess in clinical practice today.
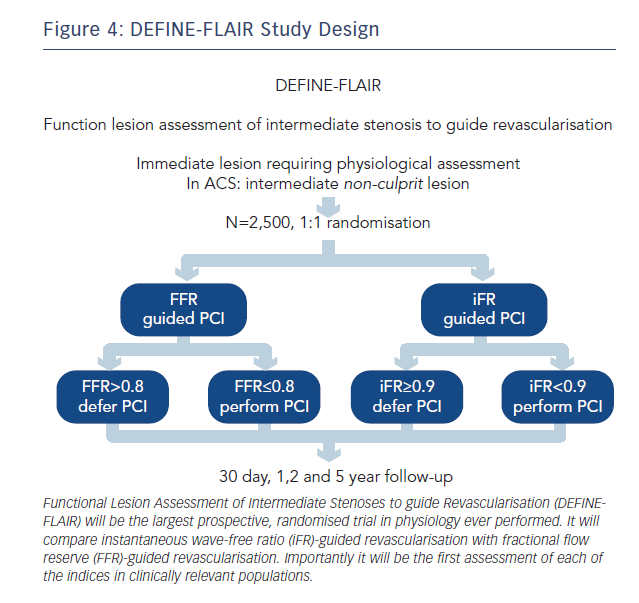
To address this, iFR clinical outcome studies have been designed to centre on clinically relevant patient populations. Their inclusion and exclusion criteria are aimed at truly capturing the patients in whom physiology is performed in clinical practice. As a result, regardless of iFR, DEFINE-FLAIR (NCT02053038, see Figure 4) and iFR SWEDEHEART (NCT02166736) will provide the first and largest prospective real-world randomised controlled trial database for FFR in clinically relevant patient populations.
In addition there are several other unique aspects of these studies that will have direct clinical impact:
- They will provide the largest prospective evaluation of physiology to guide revascularisation in non-culprit vessels in acute coronary syndrome patients.
- They will provide the largest prospective evaluation of post-percutaneous coronary intervention (PCI) physiology assessment to predict clinical outcomes.
- They will provide the largest prospective evaluation of physiology to guide multi-vessel revascularisation. Combined, these studies will include at least three times the number of patients in FAME I and FAME II combined.
- FLAIR includes over 40 centres in over 18 countries and will therefore provide the first and largest prospective comparison of physiology outcomes between different global geographical regions.
- These studies will also provide a contemporary insight into the outcomes of the major clinical patient subsets of diabetes, renal failure and hypertension.
DEFINE-FLAIR and iFR SWEDEHEART will therefore provide a definitive prospective assessment of the role of both iFR and FFR in clinical practice and address some of the major uncertainties hitherto unaddressed in this field.
Regardless of the current debate upon the physiology of basal versus hyperaemia indices the data from these studies will definitively answer if hyperaemia is still required to safely assess stenosis severity and guide revascularisation. Given the simplicity and reduced uncertainty of iFR assessment they therefore have the potential to dramatically increase the adoption of physiology, finally permitting realisation of the clear health and cost benefits of such an approach.
Integrating Physiology into the DNA of Revascularisation – A Blueprint for the Future
To date, FFR has generally been used in a binary fashion to inform the clinician if the vessel should be treated or not. The physiology of resting haemodynamics means that it is possible for indices such as iFR to provide a further dimension to the role of physiology
in the catheter lab by not only guiding when to treat but also where to treat. This is particularly useful in the increasingly prevalent subset of patients with diffuse coronary disease and tandem stenosis. In these patients iFR has the potential to reduce the prevalence of abnormal physiology post-PCI by ensuring that
the correct lesion is targeted.
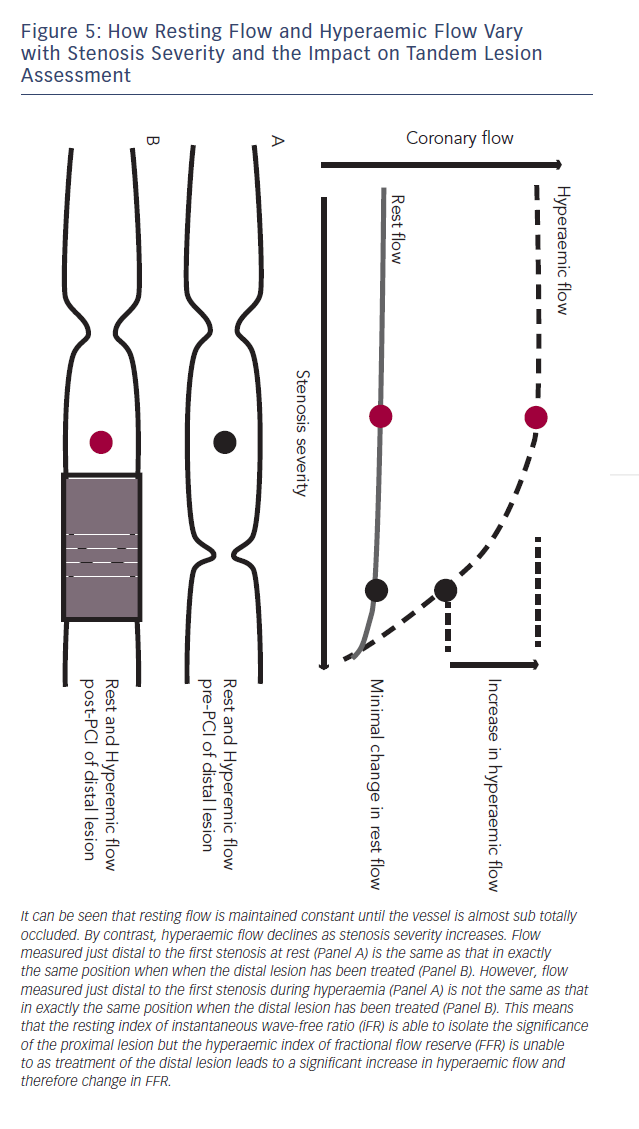
The reliance of FFR on maximal hyperaemia has prevented it from being used to isolate individual stenosis severity in vessels with tandem lesions.45,46 This is because when trying to assess the significance of a proximal stenosis the distal stenosis will blunt hyperaemic flow across the proximal lesion, therefore placement of the pressure sensor in between the two stenoses will under-estimate the severity of the proximal lesion. When the distal lesion is treated, hyperaemic flow is much higher and the FFR of the proximal vessel significantly different (see Figure 5).
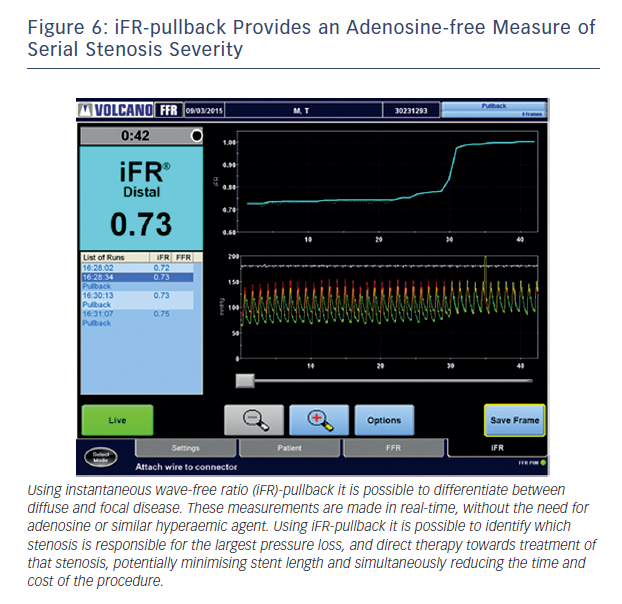
Baseline indices, by contrast, are uniquely suited to the isolation of a specific stenosis in the context of tandem lesions. This is due to the fact that baseline flow is maintained constant until stenosis grades of greater than 85 %.47 As a result, iFR is able to isolate specific stenosis severity within a vessel with multiple lesions (see Figure 6). When the distal lesion is treated, in contrast to hyperaemic flow, baseline flow does not significantly change and
therefore the pressure drop across the proximal stenosis and iFR remains similar48 (see Figure 5).
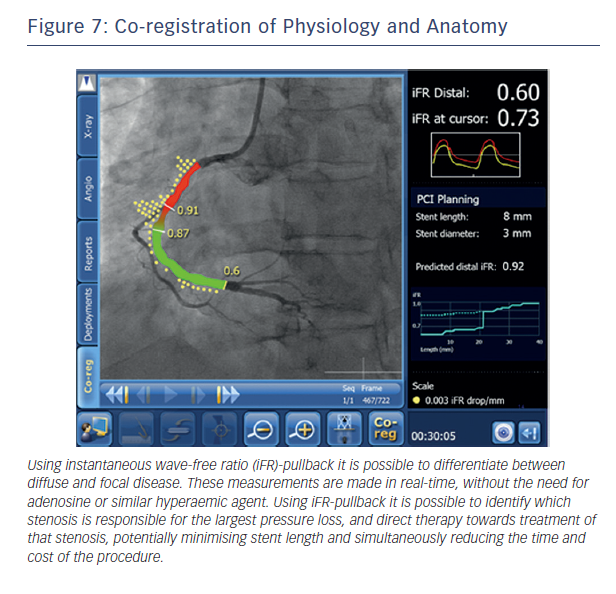
This ability to predict post-PCI iFR provides the potential to extend the role of physiology in interventional cardiology. Rather than simply informing us that a vessel is ischaemic, real-time hyperaemia-free point-to-point calculation of iFR along the entire vessel combined with co-registration with imaging will permit the possibility of virtual PCI. The clinician will therefore be able to determine (i) if a stent is required (ii) where the stent should be placed and (iii) the length of the stent to get the best haemodynamic result within a matter of seconds and remove the need for multiple hyperaemic pullback runs (see Figure 7).
Such an approach can be expected to significantly reduce stent length, procedural time and costs while simultaneously improving adoption in a patient population that is currently poorly served by FFR.
Conclusion
The routine use of physiology to guide revascularisation is unacceptably low. To improve adoption iFR has been introduced. This index is based on established physiology, can be measured within seconds, does not require hyperaemic agents and uses existing pressure wire technology. Importantly, it has the potential to extend the role of physiology by permitting accurate assessment of vessels with diffuse disease or tandem lesions. The unique characteristics of iFR physiology combined with modern imaging opens the potential of virtual PCI: for the first time allowing physiology to tell us not only if we need a stent but where and therefore how to stent to get the best outcomes for our patients.