Many advances have been made in the management of ST elevation myocardial infarction (STEMI) over the past three decades.1 This is owed to insight into role that thrombus has in the obstruction of the infarct-related artery (IRA) and the subsequent cascade of the myocardial ischaemia, cell oedema and myocardial necrosis. The institution of reperfusion therapy has revolutionised the care of patients with STEMI decreasing morbidity and mortality.2–5 This therapy, whether it be pharmacologic in the case of fibrinolysis or mechanical in the case of percutaneous coronary intervention (PCI), aims at restoring patency of the IRA and ultimately tissue perfusion. However, even with modern primary PCI, distal embolisation of thrombus is common and about a third of patients have impaired microvascular perfusion despite TIMI 3 flow in infarct vessel.6 This article will review the importance of thrombus in STEMI and approaches to management: mechanical and pharmacologic.
The Importance of Thrombus in the Pathophysiology of ST Elevation Myocardial Infarction
Mechanism of Acute Coronary Syndrome in the Formation of Thrombus
The pathophysiology of acute coronary syndrome (ACS) is rupture or erosion of the fibrous cap overlying lipid rich plaques within the arterial tree.1 This event exposes pro-inflammatory substances, ultimately resulting in platelet aggregation and formation of obstructive thrombus.1,7 Angiographic evidence of thrombus formation can be seen in more than 90 % of patients who present with ST elevation myocardial infarction (STEMI).8 Plaque rupture usually produces combination of red (cross-linked fibrin and red blood cells) and white (platelet aggregates) thrombus.9 Reperfusion therapy has become the cornerstone in the treatment of STEMI.10–13 The basis of this strategy is to restore epicardial blood flow either by the fibrinolysis of thrombus or by mechanical displacement of thrombus in the case of percutaneous coronary intervention (PCI).
The Effectiveness of Reperfusion Therapy – Early Success in Thrombus Management
The effectiveness of thrombolytic therapy has been well demonstrated in the Second international study of infarct survival (ISIS-2) study. This landmark randomised trial of 17,187 patients compared streptokinase alone, aspirin alone, the combination of aspirin and streptokinase vs neither in patients with suspected acute myocardial infarction (AMI).2 ISIS-2 demonstrated that streptokinase reduced mortality by 25 % and the combination of aspirin and streptokinase reduced mortality by 39 per cent.2 However, one of the limitations of fibrinolytic therapy is that reperfusion of the infarct artery is only successful in 50–60 % of cases.14 In comparison, primary PCI achieves TIMI 3 flow in 80–90 % of cases and meta-analyses of randomised trials show that PPCI compared to fibrinolysis reduces mortality.5,15
Microvascular vs Macrovascular Reperfusion- Implications on Clinical Reperfusion
Patency in Infarct-related Artery After Acute Myocardial Infarction and Outcome
Previously, patency of infarct-related artery after thrombolysis has been defined by the Thrombolysis in myocardial infarction (TIMI) research group.16 The TIMI system grades antegrade flow seen angiographically. Grade 0 denotes no perfusion as seen by absence of contrast flow through stenosis.16 Grade 1 flow means there is contrast seen through stenosis but the artery fails to completely fill the entirety of the artery.16 Grade 2 flow indicates complete filling of the artery with contrast past the stenosis but the rate of flow is less than that seen in a normal artery, or contrast clearance is delayed compared to that seen in a normal artery.16 Grade 3 indicates that artery fills and clears of contrast completely at a rate comparable to a normal artery.16 The open artery hypothesis that relates an improvement in survival to establishing normal flow in the IRA and hence patency of the artery has been demonstrated in previous studies.17 TIMI grade 3 is associated with a marked reduction in 30-day mortality, with an odds ratio of 0.44 (95 % CI, 0.24 to 0.79).17 There appears to be stepwise improvement in outcomes with ranging from TIMI 0–3 after primary PCI.15,18 However, TIMI flow as a prognostic tool after PPCI is less useful because more than 90 % of patients have TIMI 3 flow at end of PPCI.15
Microvascular Perfusion
There has been increasing focus on looking beyond flow in infarct artery to microvascular perfusion. ST resolution (STR) is considered a non-invasive measure of tissue perfusion.15 After PPCI, it has been shown that there was a reduction of mortality with more complete STR – in those with absent STR (<30 %) the mortality rate is 8.4 % whereas those with partial STR (30–70%) and complete STR (>70 %) have a mortality of 5.0 and 5.6 % respectively.19
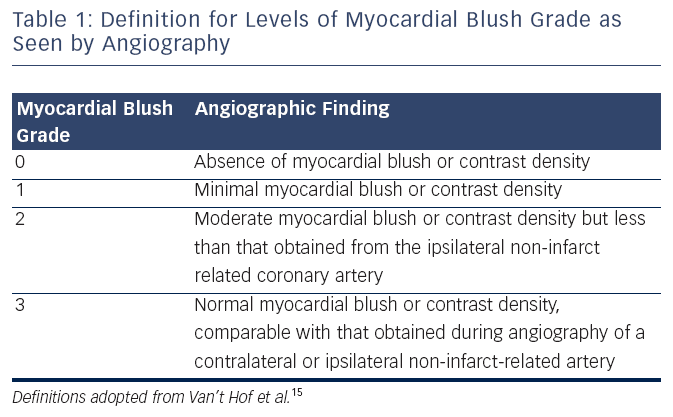
Microvascular flow can also be assessed by the myocardial blush grade (MBG), an angiographic measure of microvascular perfusion. As shown in (see Table 1) MBG is graded from 0 (absence of blush) to 3 (normal myocardial blush). Blush assessment requires a longer than average cine run to determine if blush clears.15
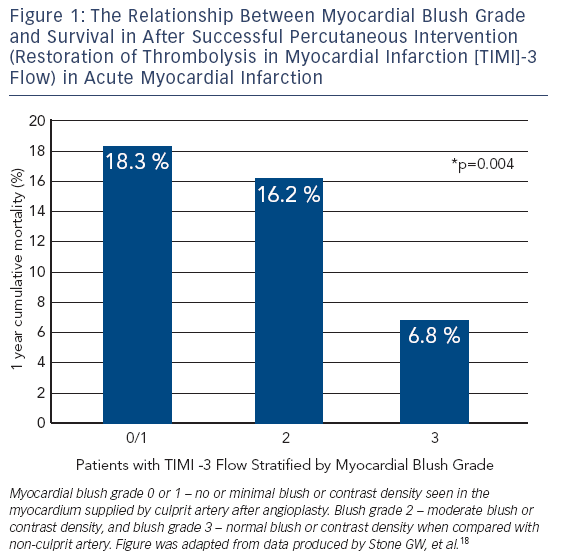
MBG has been shown to be an independent predictor of ST segment resolution, Killip Class after primary PCI and mortality.15 Compared to MBG of three, an MBG of zero or one has an eight-fold higher risk of long-term mortality (3 vs 23 % at two years, p< 0.0001).15 Interestingly, in the same study up to 67 % of patients with TIMI 3 flow had MBG of 0 or 1 which again suggests that epicardial blood flow does not necessarily imply tissue level perfusion.15 Even among those with TIMI 3 flow after angioplasty, an MBG grade of 0 or 1 may be associated with an increase for mortality (relative risk 4.7; 95 % CI 2.3 to 9.5; p< 0.001) (see Figure 1).18,20 This data has led to new paradigm that TIMI 3 flow is not enough; we must find methods to improve microvascular perfusion during PPCI.
Abnormal tissue perfusion in the presence of a patent epicardial artery is a phenomenon which is referred to as “No Reflow”.6 TIMI flow of less than three after an artery has been opened during PPCI is the most common finding which can be associated with no reflow. It is also an independent predictor of long-term cardiac death (relative risk [RR] 5.25, 95 % confidence interval [CI] 1.85 to 14.9, p=0002).21
Pharmacological Strategies in Thrombus Management
The Role of Adjunctive Glycoprotein IIb IIIa Inhibitors (GP IIb IIIa Inhibitors): Intracoronary Abciximab
Localised directed intracoronary (IC) administration of Abciximab in the infarct-related artery has attracted some recent research interest. There is theoretical advantage to this route of administration of providing a higher concentration of active drug at the site of thrombus given that Abciximab has a short plasma half-life. The intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction (INFUSE AMI) (N=452) study was a 2x2 factorial design randomising patients with STEMI on a background of dual antiplatelet therapy and Bivalarudin to thrombectomy plus IC abciximab (via clearway catheter), aspiration thrombectomy without IC abciximab, no aspiration thrombectomy plus IC abciximab and no aspiration thrombectomy plus no IC abciximab. This study showed a 2.8 % reduction in infarct size (p= 0.03) but also a numerical but not significant increase in TIMI major bleeding (2.2 vs 0.5 %; p =0.40).22 The significantly larger Abciximab intracoronary vs Intravenous drug application in ST-elevation myocardial infarction trial (AIDA STEMI) trial (N= 2065) comparing IC bolus (via guide catheter) vs intraveous abciximab in patients with STEMI showed no difference in the composite primary endpoint (all cause mortality, recurrent infarction or new congestive heart failure at 90 days (7.0 vs 7.6 %; odds ratio [OR] 0.91; 95 % CI 0.64-1.28; p=0.58).23
The primary difference in the two trials is lack of abciximab in control group in INFUSE AMI. At the current time, the evidence does not support routine use of IC abciximab but future large scale randomised trials are needed if locally directed therapy (i.e. via clearway catheter) improves clinical outcomes.
Covered Stents
There has been recent exploration of a bare metal stent (BMS) platform covered with a polyethylene terephthalate mesh (Mguard™ stent) that aims to trap thrombus and hence prevent distal embolisation.24 Therehas been one multicentre randomised study (n=433) comparing the efficacy of Mguard stent to conventional stents in STEMI. Both bare metal stents (BMS) or drug eluting stents (DES) at the operators discretion were allowed in the control arm. The primary outcome of complete STR post-procedure was significantly better in the patients randomised to the MGuard stenting arm compared with conventional stenting (57.8 % vs 44.7 %, absolute difference 13.2 %; 95 % CI 3.1 %–23.3 %; p=0.008). Limitations of the Mguard is coverage of side branches with mesh and bulkiness of device. The MASTER II trial is underway (N=1114), and is a larger trial testing Mguard vs BMS or DES in STEMI with a primary outcome of ST resolution. Ultimately, larger clinical outcome trials are needed to determine if this strategy of trapping thrombus with a mesh covered stent improves clinical outcomes and it would be optimal to have a drug eluting version to avoid restenosis.
Thrombectomy – Manual and Mechanical
The rationale of thrombectomy is that if one can remove thrombus prior to deploying stent, there will be improvement in the myocardial blush grade and the risk of distal embolisation and no reflow can be reduced.25 There are two major types of thrombectomy – mechanical and manual aspiration.
Manual Thrombectomy
Manual thrombectomy uses very simple devices that are essentially long tubes with syringes on the end. The Thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction study (TAPAS) trial was a single-centre trial (N=1071) that showed that in patients with STEMI, routine manual thrombectomy compared to PCI alone reduced impaired microvascular perfusion (primary outcome MBG zero or one) by 35 % (p< 0.001) and a trend toward reduced in cardiac mortality at 30 days (2.1 % versus 4.0 %; risk ratio, 0.52; 95 % CI, 0.26 to 1.07, p=0.07). At one-year follow-up this difference in cardiac death became statistically significant (3.6 % in thrombus aspiration group vs 6.7 % in the PCI alone group; hazard ratio (HR) 1.93; 95 % CI 1.11-3.37; p=0.02).26 Subsequent meta-analyses showed reductions in mortality but this was driven by the TAPAS trial.27 Based on the TAPAS trials, both the European Society of Cardiology (ESC) and the American College of Cardiology (ACC) provided a class IIa recommendation for routine use of manual aspiration in primary PCI.12,28
The most recent and largest trial, the Thrombus aspiration in ST-elevation myocardial infarction in Scandinavia (TASTE) trial, a multicentre study randomising 7244 patients to thrombus aspiration versus PCI alone.29 The enrolment and randomisation was done within the infrastructure of the Swedish coronary angiography and angioplasty registry (SCAAR).29 Based on actual mortality rates in the Swedish registry, there was an expected 452 events to have an 80 % power to detect a 30 % relative risk reduction (RR).29 There was no significant difference in the primary outcome of all cause mortality at 30 days between thrombus aspiration plus PCI vs PCI alone (2.8 vs 3.0 %, hazard ratio 0.94, confidence interval [CI] 0.72 to 1.22, p=0.63).29 There were trends towards reduction in hospitalisation due to recurrent MI (0.5 % vs 0.9 % respectively; HR 0.61; 95 % CI, 0.34–1.07; p=0.09) and stent thrombosis (0.2 % vs 0.5 % respectively; HR 0.47; 95 % CI 0.20-1.02, p=0.06).29 TASTE had less than half the original number of planned events and so was underpowered formodest but clinically important reductions (20–30 % RRR) in all cause mortality. As a result, further data is needed.
The ongoing randomised trial of routine aspiration Thrombectomy with PCI versus PCI alone in patients with STEMI undergoing primary PCI (TOTAL) is an event driven trial that will recruit 10,700 patients. The primary outcome will be cardiovascular death, MI, cardiogenic shock and class IV heart failure up to 180 days.30 The hypothesis of the trial is that by reducing thrombus burden at site of stent implantation, thrombectomy can prevent MI and stent thrombosis and by preventing no reflow, thrombectomy can prevent cardiogenic shock, heart failure and death. The TOTAL trial will definitively answer the question of whether routine aspiration thrombectomy reduces important clinical outcomes in primary PCI.
Mechanical Thrombectomy
The most commonly used device employed for mechanical thrombectomy is the Angiojet rheolytic thrombectomy (RT) catheter.31 This device uses high velocity saline jets to break up thrombus and active suction to remove thrombus. There have been to randomised trials comparing Angiojet to conventional PCI in STEMI but have yielded conflicting results. The AngioJet rheolytic thrombectomy in patients undergoing primary angioplasty for acute myocardial infarction (AIMI) trial (n= 480) showed a 27 % increase in infarct size (p=0.03), no difference in STR or MBG and increase in mortality with routine use of Angiojet.32 The AngioJet rheolytic thrombectomy before direct infarct artery stenting with direct stenting alone in patients with acute myocardial infarction (JETSTENT) trial (n=501) showed that patients receiving routine RT before direct stenting (DS) compared with DT alone had a 7 % increase in STR (p= 0.04) but no significant improvement in infarct size. Unexpectedly the overall major adverse cardiovascular events (MACE) rates at six months were lower in the RT before DS compared to DS alone (11.2 % versus 19.4 %; p=0.011). The primary difference in the trials is the selection of patients with high thrombus burden in the JETSTENT vs all comers. It may be that the Angiojet is beneficial in those with large thrombus burden and not in those patients with minimal thrombus.
A recent optical coherence tomography (OCT) trial suggests that the Angiojet when compared to manual thrombectomy may be more effective at thrombus removal.33 Future large-scale trials are needed to determine the effect of the modern Angiojet on clinical outcomes in the subset of patients with high thrombus burden after wire crossing.
Intracoronary Thrombolysis Prior to Manual Thrombectomy
There has only been one randomised study investigating the effect of IC thrombolytic delivery prior to aspiration thrombectomy. The Delivery of thrombolytIcs before thrombectomy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention (DISSOLUTION) trial (n=102) compared IC thrombolytic delivery (urokinase at 200, 000 U) via a microcatheter prior to aspiration thrombectomy compared with IC normal saline control via microcatheter prior to aspiration thrombectomy in patients with large thrombus.34 It showed that patients treated with IC thrombolytic upfront prior to aspiration thrombectomy compared to control showed a higher rate of TIMI 3 flow (90 vs 66 %; p=0.008), higher rate of MBG 2 or 3 (68 vs 45 %; p=0.028) and higher rate of STR >70 % (82 vs 55 %, p=0.006) It also showed a significantly lower rate of MACE at six months in the upfront IC thombolytic group compared to control (6 % vs 21 %; p=0.044) but this was entirely driven by a reduction in re-hospitalisation for heart failure. Thrombolysis prior to thrombectomy allowed greater volume of aspirate from manual thrombectomy. Further larger randomised trials are needed to validate these findings and test safety and efficacy of IC lytics as an adjunct to PCI.
Conclusion
Rapid reperfusion therapy has led to marked to reductions in mortality in STEMI. However, therapies focused at preventing thrombus embolisation have failed to show improvements in mortality but so far trials have been underpowered. The largest trial of manual thrombectomy, the TOTAL trial, will inform us of the effect of routine manual thrombectomy on clinical outcomes in STEMI.