While provisional stenting has remained the preferred strategy for majority of bifurcation lesions, controversies still exist regarding when and how to use complex techniques. Most of randomised studies comparing simple and complex approaches have focused on selected populations, included both ‘true’ and ‘non-true’ bifurcation lesions, used first-generation drug eluting stents (DES) and favoured the crush technique performed in a suboptimal manner (i.e. no use of non-compliant balloon post-dilatation).1–3 Currently, there are increasingly more reasons to believe that optimally performed two-stent techniques, with the use of newgeneration DES, may provide similar or better results compared with the simple techniques.4–6 Studies have shown a trend towards improved clinical and/or angiographic outcomes with a two-stent strategy, when used in bifurcations with large side branches (SBs).7–10 Two of the studies also revealed that at follow-up, the SB diameter stenosis was higher, and restenosis was more frequent with provisional stenting.8,9 Moreover, these studies revealed that in true bifurcation lesions the rate of crossover from simple to complex strategies might be as high as 16 %.7 Furthermore, in an all-comers population of patients treated with current-generation DES, a provisional strategy bears a risk of intraprocedural SB closure exceeding 8 %.6
Limitations of Currently Used Stents
Currently used stents were designed to treat lesions in simple anatomy of straight vessels, not at the bifurcation site, where often major stent deformations are necessary to adequately cover the target territory. This raises the following concerns:
- Double-stent techniques are difficult, 1 more complex to perform than single-stent procedures, thus the outcomes are more operator dependent.
- Stent distortion, which is common in complex techniques, may lead to strut fractures and malapposition, SB jailing or inability to fully cover the SB ostium.11,12
- Most studies on currently used stents have revealed an increased risk of periprocedural MI with two-stent techniques,2,3 in comparison with simple procedures, while being equally effective, and safer than complex ones.
These issues provide a potential role for dedicated bifurcation stents. These devices must be easy to use, effective in various lesion morphologies and safe.
Development of Dedicated Devices
Currently, different dedicated devices are used or being developed and classified according to different rules. A simple classification is based on the primary role of the stent, i.e. main branch stents (MBS), dedicated to treating the main vessel; and side branch stents (SBS), dedicated to treating and protecting the SB. MBS allows for both simple and complex strategies, as needed. This group includes the following devices: AxxessTM (Biosensors Europe SA), BiOSS® and BiOSS LIM® (Balton), Nile CroCo® and Nile PAX® (Minvasys), STENTYSTM (STENTYS SAS), Xience SBATM (Abbott Vascular), Twin RailTM (Invatec/Medtronic), TAXUS® PetalTM (Boston Scientific) and others. Contrary to expectations, most are not easy to use, require wide operator experience, and may themselves cause some devicespecific technical problems. Therefore, many of these devices have not entered routine clinical practice. A guiding catheter larger than 6F is necessary to implant the Axxess, Xience SBA, and TAXUS Petal stents, wire wrapping may make implantation of some devices (e.g. Axxess, Nile CroCo, Twin Rail, Petal) demanding, device self-alignment is often incomplete (e.g. Xience SBA, TAXUS Petal), and active, controlled rotation may be impossible (e.g. Xience SBA).
Axxess Stent
The Axxess stent is a 150 micron-thick strut, self-expanding nitinol stent, covered with Biolimus A9TM (Biosensors International), a drug released from biodegradable polymer. According to MADS (main, across, distal, side) classification, the stent belongs to the M category (main proximal first), as it covers the only the proximal main branch (MB) up to the carina level.13 While recrossing into the SB is not necessary, this technique often requires additional implantation of one or two stents to treat the lesions located in distal MB and SB. The procedure itself requires experience and precision when nesting the device at the carina. In the AXXESS Plus pivotal study, device success rate was 93.5 %, and 80.9 % of the patients received additional stents.14 The overall rate of major adverse cardiac events (MACE) at 6 months was 11.2 %, with no cases of acute or subacute stent thrombosis (ST), although late ST was reported in 2.2 % of the treated patients. The recently published COBRA trial reported on 40 patients with true bifurcation lesions randomly assigned to treatment with the Axxess and additional BioMatrixTM (Biosensors International) stents or a culotte technique using XIENCETM (Abbott Vascular).15 Implantation of the Axxess stent resulted in a significantly larger lumen in the proximal MB after the procedure and at follow-up, and in a lower angiographic late lumen loss (p=0.05). Both strategies resulted in good clinical outcomes with a rate of 10 % MACE at 1 year.
BiOSS Stents
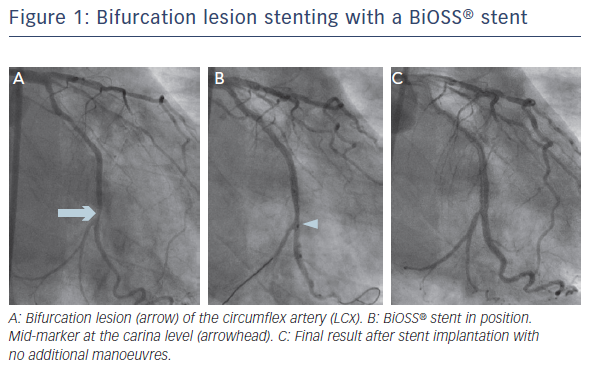
The other device within MBS group is the BiOSS stent, for which there are two types: the older, paclitaxel-eluting BiOSS Expert® (Balton) and sirolimus-eluting BIOSS LIM. Both drugs are released from biodegradable polymer. The stent was designed to respect the fractal geometry of bifurcation, hence the proximal region has a larger diameter than the distal (the proximal/distal diameter ratio is 1.15–1.3). Both regions are joined by two connecting struts (mid zone). The stent is mounted on a special stepped delivery Bottle® balloon (Balton). The balloon mid marker, which shows the mid zone, allows for adequate stent positioning. According to the MADS classification,13 the stent belongs to ‘A’ group (provisional stenting). Once the mid marker is positioned at the tip of the carina, the stent is fully opened and no or minimal carina shift towards the SB should occur. An SB stent may by implanted as needed in either the T/T with protrusion or culotte mode.16 The ease of use is the major advantage of the BiOSS stent, whereas the lack of the coverage of the SB ostium is a major limitation. An example of bifurcation lesion stenting with a BiOSS stent is shown in Figure 1.
In the POLBOS (Polish Bifurcation Optimal Stenting) I randomised study, the BiOSS Expert stent was compared with regular DES.17 A total of 243 patients were enrolled and randomised (1:1) to receive either treatment. Additional SB stenting was required in 10 % of cases in both groups. At 12 months, cumulative incidence of MACE was similar in both groups, but the target lesion revascularisation (TLR) rate was significantly higher in the BiOSS group (11.5 versus 7.3 %; p=0.02). In the POLBOS II study, patients were randomised to either the BiOSS LIM group (n=102) or to the conventional DES group (n=100).18 Contrary to expectations, SB stenting was required in a greater proportion of the BiOSS group (8.8 %) than the provisional group (7 %), but with no statistical significance. At 12 months, the cumulative incidences of MACE and TLR were similar in both groups: 11.8 versus 15 % (p=0.08) and 9.8 versus 9 % (p=0.8) for BiOSS LIM and DES groups, respectively. The BiOSS LIM stent was also examined in the prospective international registry enrolling 74 patients with distal left main stenosis.19 At 12 months, the rate of MACE was 9.5 % without cardiac death or definite stent thrombosis. TLR and MI rates were 6.8 % and 2.7 %, respectively.
Advantage of Main Branch Stent-Dedicated Devices over Provisional Stenting with Conventional Drug-eluting Stents
To date, no studies have shown a clear advantage of MBS-dedicated devices over provisional stenting with the use of conventional DES. Apart from the BiOSS stent, devices are not easy to use and require some extra device-specific skills. Another weakness of this technology is a limited range of device length, thus multiple stents may be required for implantation into the MB.
The primary role of SBS devices is to treat and protect the SB during MB stenting. This allows for the use of any DES length and diameter to treat the main vessel lesion, both proximally and distally to the SB, which is an undisputable advantage of this approach. In contrast, the wide selection of DES covered with different polymers and drugs contributed to the current bare metal designs for SBS. This strategy a priori necessitates use of the two-stent technique, with one possible exemption of Medina 0,0,1 lesions.20
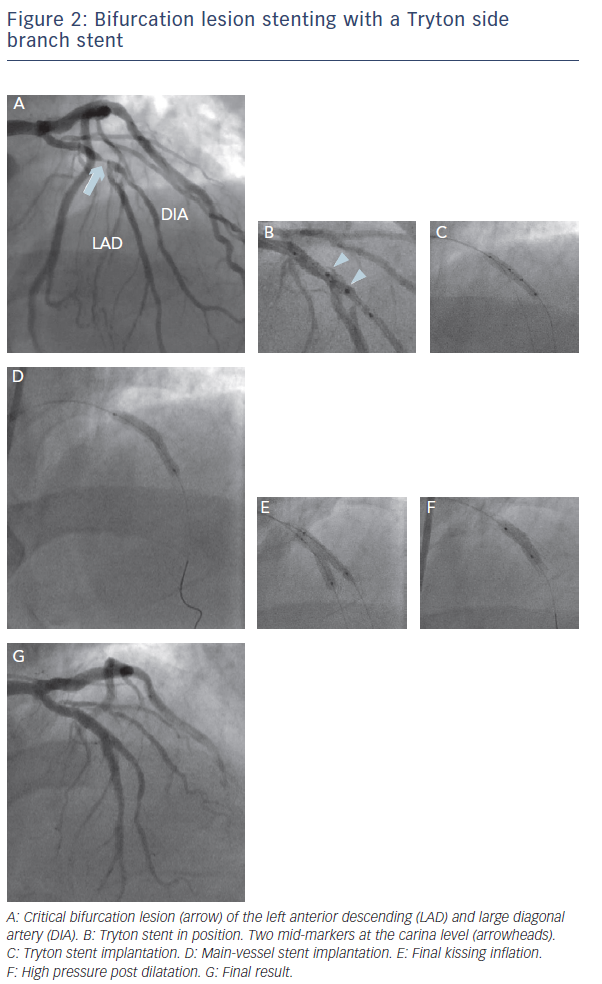
The Tryton SB stent (Tryton Medical) is the most widely studied dedicated device. This is a thin-strut, cobalt-chromium bare metal stent, and consists of three zones: a distal, slotted-tube SB zone, for treatment and protection of the SB; a transition zone, to be positioned at the SB ostium; and an MB zone with two wedding bands and three undulating fronds minimising the amount of metal. The stent is mounted either on a straight or on a stepped balloon, to respect the fractal geometry of coronary bifurcation. According to the MADS classification, the technique with the Tryton SB stent belongs to inverted ‘A’ category (inverted culotte). An example of bifurcation lesion stenting with a Tryton SB stent is shown in Figure 2.
The stent has been examined in multiple registries and in one large randomised trial. In a large E-Tryton 150/Benelux registry a total of 302 patients were enrolled.21 Technical and procedural success rates reached 98.0 % and 94.4 %, respectively. The cumulative 6-month MACE rate was 6.4 % with no deaths, 4.7 % MI events, and 3.4 % target lesion revascularisation events. Only one case of stent thrombosis was reported.
In the largest multicentre, randomised study to date, the TRYTON Pivotal Trial, the two-stent strategy with the use of Tryton SB stents in >700 patients with true bifurcation lesions, did not show clinical noninferiority to provisional main vessel stenting with regular DES, mainly due to a small excess of a study-defined periprocedural MI events.9 This could be explained by the inclusion of relatively small SBs in the trial. Actually, 60 % of patients did not meet the inclusion criterion of SB diameter >2.5 mm by visual estimate, which corresponded to the 2.25 mm determined in the quantitative coronary angiography (QCA) core laboratory analysis. A post-hoc analysis identified a strong interaction in the occurrence of the primary clinical endpoint (target vessel failure), showing lack of benefit of the complex strategy in smaller SBs and potential benefit in larger ones.10 This confirms the findings reported in recent randomised studies and registries with conventional stents implanted in bifurcation lesions with large SBs.6–8
Recently, the Tryton Confirmatory Study has been reported,22 a study designed to confirm the results of the post-hoc analysis of the TRYTON Pivotal trial. A total of 133 patients with bifurcation lesions containing large SBs (≥2.25 mm, confirmed by QCA) were enrolled. The primary endpoint was non-inferiority with regard to periprocedural MI as compared with the provisional cohort of the previous Tryton study. In patients with large SBs, periprocedural MI rate (10.5 %) was lower than in the provisional group in the TRYTON Pivotal trial (11.9 %). This result has met the non-inferiority primary endpoint.22 Although no study revealed explicit superiority of the Tryton SB stent over a simple technique with current-generation DES, one should keep in mind that the stent is a bare metal device. Further improvement in clinical outcomes should be expected once the Tryton SB stent is designed to be covered with a potent antiproliferative drug.
Advances in Bifurcation Stenting
Recently there have been major advances in bifurcation stenting. The widespread use of intravascular imaging, routine usage of the proximal optimisation technique and final kissing balloon inflations with noncompliant balloons improved markedly the clinical outcomes of percutaneous coronary intervention procedures. Bifurcation-dedicated technology needs much further improvement. Devices and techniques that require special operator skills or the use of unconventional accessories, will not likely be adopted. Simple, thin-strut, drug-eluting devices, with a high capacity for adaptation to various anatomical patterns will be eagerly accepted. Many interventional cardiologists instinctively feel that bifurcation-dedicated stents should finally overcome numerous limitations of conventional DES. However, none of the clinical studies reported thus far showed any advantage of this approach compared with simple provisional strategies. Perhaps the key is the proper selection of patients and lesions. Three anatomical factors seem to play a major role, and all involve an SB: the importance of this vessel, the extent of atherosclerotic disease and ease of SB access. The loss of a vessel supplying substantial amount of myocardium will always lead to a large periprocedural MI,23 which may worsen patients’ prognosis.24 Thus, it seems that the presence of a large SB, especially with significant stenosis at the ostium, should be an explicit indication for more complex techniques, with possible use of simple dedicated devices. Recent studies on systematic twostent strategies in large-calibre true bifurcations, with the use of current-generation conventional DES6–8 or dedicated stents9,10,19,22 seem to confirm this hypothesis. In addition, complexity of SB stenosis plays an important role. The authors of the Definitions and Impact of Complex Bifurcation Lesions on Clinical Outcomes After Percutaneous Coronary InterventIon Using Drug-eluting Stents (DEFINITION) study found that for complex bifurcation lesions with severe and extensive disease in the SB, two-stent techniques were associated with lower rates of in-hospital MACE and 1-year cardiac death as compared with provisional stenting.25
Conclusion
While the provisional technique still remains a treatment of choice for bifurcation lesions, the use of complex two-stent strategy, with or without dedicated stents, may be a better option in cases in which an SB is large or diseased or if there is difficulty in accessing this vessel.