When President Dwight Eisenhower suffered a heart attack on the golf course in Colorado in 1955, there were limited treatment options. He was treated with morphine, amyl nitrate, oxygen, heparin and six weeks of bed rest. Mortality for patients hospitalised with a heart attack at that time was about 25 %. When reperfusion therapy with streptokinase was introduced with the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infartol Miocardico (GISSI) and Second International Study of Infarct Survival (ISIS-2) trials in the late 1980s, hospital mortality in the control groups was about 13 %. Over the 25 years since that time, with the introduction of new generation fibrinolytic agents, primary angioplasty and stenting and new antiplatelet and anticoagulant therapies, short-term mortality has fallen to about 2–3 % in recent randomised trials. This is one of the most impressive success stories in modern medicine.
In this review, we plan to provide an update on current optimum therapies for patients with ST-segment elevation myocardial infarction (STEMI). We will focus on management with primary percutaneous coronary intervention (PCI), since this has become the preferred reperfusion strategy for STEMI patients. We will concentrate on four topics based on their importance and the fact that there are some differences of opinion and controversy regarding optimum management in these areas. The topics and questions we will discuss are the following:
- What is the optimal adjunctive anti-thrombotic therapy with primary PCI?
- How can we prevent stent thrombosis after stenting for STEMI?
- How should we manage coronary thrombus at the time of PCI?
- What is the optimum reperfusion strategy for STEMI patients presenting at non-PCI hospitals?
Optimal Adjunctive Anti-thrombotic Therapy with Primary Percutaneous Coronary Intervention
When primary angioplasty was first introduced as a reperfusion strategy in the early 1980s, the standard anti-thrombotic therapy consisted of aspirin and unfractionated heparin. Glycoprotein IIb/IIIa platelet inhibitors (GPI) were introduced in the late 1990s and were shown to reduce recurrent ischaemic events following primary PCI and meta-analyses suggested that these agents may reduce short–term mortality. A few years later clopidogrel was introduced and was shown to reduce the frequency of stent thrombosis and other adverse events. So in the early 2000s, triple antiplatelet therapy with aspirin, GPI and clopidogrel combined with unfractionated heparin became the standard anti-thrombotic therapy with primary PCI.
It was soon recognised that this combination of drugs was associated with a high incidence of bleeding. It was uncertain whether GPI were necessary when clopidogrel was used, since the studies documenting the efficacy and safety of GPI were performed before the introduction of clopidogrel. At the same time, bivalirudin, a direct thrombin inhibitor, was introduced and was found to have advantages over unfractionated heparin. Large trials in patients with stable angina and acute coronary syndromes (ACS) undergoing PCI had shown less bleeding and less thrombocytopenia and similar major adverse cardiovascular event (MACE) rates with bivalirudin compared with unfractionated heparin.
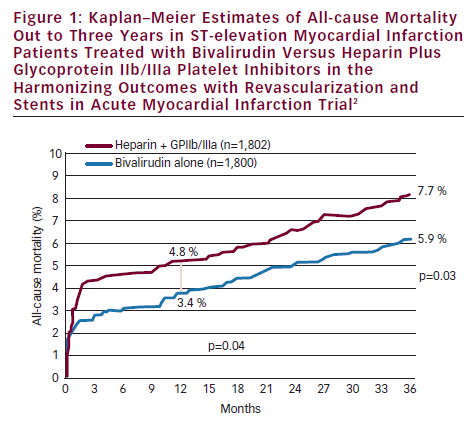
This set the stage for the Harmonizing outcomes with revascularization and stents in acute myocardial infarction (HORIZONS-AMI) trial.
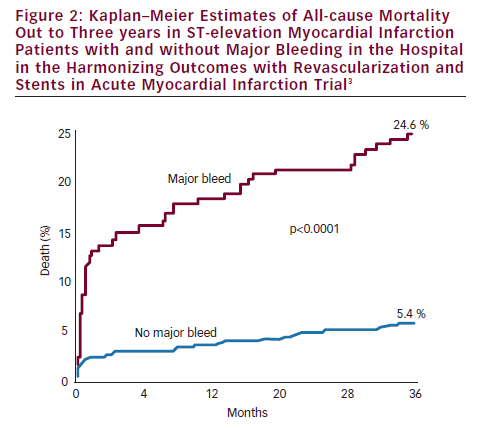
The HORIZONS-AMI trial randomised STEMI patients undergoing primary PCI to bivalirudin versus unfractionated heparin plus GPI, with both groups receiving aspirin and clopidogrel.1 The results showed similar 30-day MACE rates in both groups, but bivalirudin was associated with a lower frequency of major bleeding (4.9 versus 8.3 %, p<0.0001) and net adverse clinical events (NACE [MACE or major bleeding]) (9.2 versus 12.1 %, p=0.005).1 The trial was not powered to detect mortality differences, but somewhat unexpectedly, 30-day mortality was found to be significantly lower with bivalirudin than heparin plus GPI (2.1 versus 3.1 %, p=0.048). This benefit persisted and increased over time and at three years bivalirudin compared with heparin plus GPI was associated with lower rates of mortality (5.9 versus 7.7 %, p=0.03) and reinfarction (6.2 versus 8.2 %, p=0.04) (see Figure 1).2 The lower mortality rates have been attributed to lower rates of major bleeding, since major bleeding in the acute phase of HORIZONS-AMI was associated with much higher three-year mortality rates (24.6 versus 5.4 %, p<0.0001) (see Figure 2).3 There are several potential reasons why patients with major bleeding may have higher mortality. Mortality can result directly from the bleeding episode. Bleeding can precipitate ischaemia due to increased demand and bleeding can result in discontinuation of anticoagulant and antiplatelet therapies resulting in ischaemic events or stent thrombosis. There can be adverse effects from transfusions, which can lead to mortality. There also are co-morbidities associated with bleeding which can contribute to the association of major bleeding and mortality, but in the HORIZONS-AMI trial the association of bleeding with mortality remained highly significant after adjusting for co-morbidities.
Despite these benefits, the HORIZONS-AMI trial did reveal some limitations with the use of bivalirudin in STEMI patients. Within the first 24 hours after PCI there was a significantly higher incidence of stent thrombosis with bivalirudin compared with heparin plus GPI (1.5 versus 0.3 %, p<0.001). There was some catch-up from one to 30 days with more stent thromboses in the heparin group, so that the overall stent thrombosis rates at 30 days were higher with bivalirudin, but the differences were no longer significant (2.7 versus 1.9 %, p=0.33). The frequency of acute stent thrombosis with bivalirudin was less when patients were loaded with 600 mg clopidogrel and when patients were given a heparin bolus pre-randomisation. Although acute stent thrombosis is a problem with bivalirudin alone, the impact of acute stent thrombosis on mortality is much less than the impact of major bleeding. Hospital mortality at 30 days in HORIZONS-AMI in patients with definite stent thrombosis was 8.7 % (5/57) and in patients with major bleeding was 10.9 % (26/238). Since there were many more patients with major bleeding than stent thrombosis (238 versus 57), the number of deaths from major bleeding was much more than from stent thrombosis (26 versuss five).1 Based on these data, the new optimal anti-thrombotic therapy for STEMI treated with primary PCI became aspirin, clopidogrel and bivalirudin.
The next and most recent advance in anti-thrombotic therapy with primary PCI has come with the introduction of the new generation antiplatelet agents, prasugrel and ticagrelor. The Trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction (TRITON-TIMI 38) trial compared prasugrel with clopidogrel in patients with acute coronary syndromes (ACS). In the STEMI cohort of this trial, at 450 days, prasugrel was associated with a significant reduction in cardiovascular death, re-infarction or stroke (10.0 versus 12.4 %, p=0.02) and a significant reduction in stent thrombosis (1.6 versus 2.8 %, p=0.02) with no increase in major (non-coronary artery bypass graft [CABG]) bleeding.4 However, in the overall cohort prasugrel was associated with a significant increase in major (non-CABG) bleeding and a four-fold increase in CABG related bleeding. The Platelet inhibition and patient outcomes (PLATO) trial compared ticagrelor with clopidogrel in patients with ACS. In the STEMI cohort of this trial ticagrelor was associated with a reduction in cardiovascular death, reinfarction or stroke (9.4 versus 10.8 %, p=0.07), a reduction in stent thrombosis (1.6 versus 2.4 %, p=0.03) and lower mortality (4.9 versus 6.0 %, p=0.04) with no increase in major bleeding.5
Both prasugrel and ticagrelor appear to have major advantages over clopidogrel. Both have rapid onset of action with full platelet inhibition. Ticagrelor has more rapid offset of action, potentially facilitating early bypass surgery when necessary, but current recommendations are to postpone surgery for five days after discontinuation of the drug, so there does not appear to be a major clinical advantage. Prasugrel has more CABG-related bleeding but has a very impressive reduction in stent thrombosis. Ticagrelor appears to have a mortality advantage, but the drug must be given twice a day and there is still concern about the unexplained lack of efficacy in the US cohort. Despite some limitations, these drugs represent a significant advance over clopidogrel. So the new paradigm for optimal anti-thrombotic therapy for STEMI patients treated with primary PCI consists of aspirin, prasugrel or ticagrelor and bivalirudin, with GPI reserved for very large thrombus burden, stent thrombosis or no-reflow. The rapid onset of action with full platelet inhibition with both prasugrel and ticagrelor could potentially alleviate the problem of acute stent thrombosis in the first 24 hours when bivalirudin is used without GPI. Because of the high risk of CABG-related bleeding with prasugrel, there is still disagreement whether prasugrel should be given up front or after angiography in STEMI patients targeted for primary PCI. Although the need for emergency surgery for failed PCI is rare, there are a small number of STEMI patients who are triaged to emergency surgery rather than PCI.
Prevention of Stent Thrombosis Following Stenting for ST-elevation Myocardial Infarction
The Primary angioplasty in myocardial infarction (PAMI) and Controlled abciximab and device investigation to lower late angioplasty complications (CADILLAC) trials documented that the use of coronary stents compared with balloon angioplasty alone (PTCA) with primary PCI for STEMI reduced target vessel revascularisation (TVR) and target vessel occlusion at six to 12 months, but comparison of outcomes beyond six to 12 months was not evaluated. When drug-eluting stents (DES) were shown to further reduce TVR compared with bare metal stents (BMS) with elective PCI, they were rapidly adopted for use in STEMI patients and meta-analyses showed that DES reduced TVR with no difference in mortality, reinfarction or stent thrombosis (ST) at one year. However, in 2006 there were a number of disturbing reports regarding the increased frequency of very late ST with DES compared with BMS. In response to this the US Food and Drug Administration (FDA) created an advisory panel which reviewed available data and concluded that DES used ‘on-label’ with elective PCI were associated with a small increased risk of very late ST, but there was no evidence of increased risk of death or myocardial infarction. The panel warned that ‘off-label’ use of DES may increase the risk of ST, myocardial infarction and death. Observational data from the Thoraxcenter and others found that the risk of ST was highest when stents were implanted ‘off-label’ in STEMI patients, and the risk of very late ST in patients with acute coronary syndromes (ACS) was higher with DES than BMS.6 Pathological studies had documented that healing was delayed with DES, and Vermani and colleagues found in post-mortem studies that when DES were implanted in STEMI patients compared with elective stenting, there was worse healing with more uncovered stent struts and more inflammation.7 It had also been shown that late acquired stent malapposition was much more frequent with DES than with BMS in STEMI patients, and it has been recently shown that late stent malapposition is associated with an increased incidence of very late stent thrombosis.8 For all these reasons, there has been great concern about the risk of very late stent thrombosis when DES are used in STEMI patients.
The HORIZON-AMI trial (in addition to evaluating bivalirudin) was designed to evaluate the long-term safety and efficacy of DES (paclitaxel-eluting stents [PES]) versus BMS in STEMI patients.9 The primary efficacy endpoint, target lesion revascularisation (TLR), was significantly better with PES at one year (4.5 versus 7.5 %, p=0.002). At three years there was no difference between PES and BMS in the safety endpoints of MACE (13.6 versus 12.9 %, p=0.66) and ST (4.8 versus 4.3 %, p=0.63).2 However, there was a trend for increased frequency of very late ST from one to three years with DES (1.7 versus 0.9 %, p=0.12).2 We found in our large observational database with stenting for STEMI that very late ST was significantly more frequent with first generation DES compared with BMS at one to five years (6.9 versus 1.7 %, p=0.0002) (see Figure 3).10 We also found that the cumulative frequency of ST continues to increase out to 11 years with BMS and out to five years with DES. These observations and the observations of others have provided convincing evidence that the use of first-generation DES for STEMI patients are associated with some increased risk of very late ST.
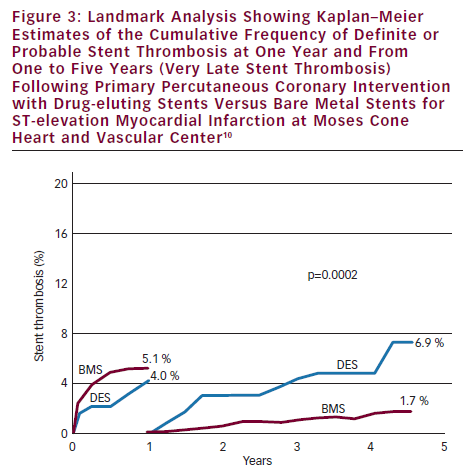
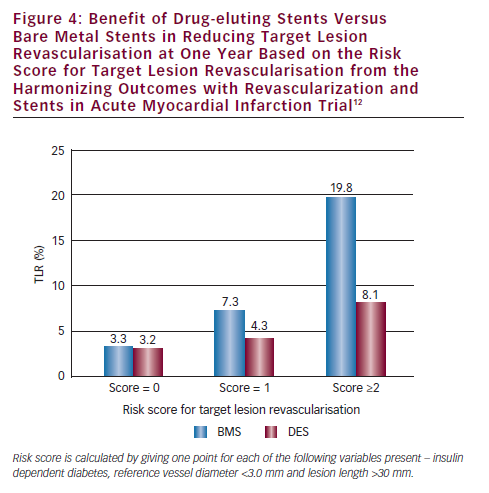
How can we prevent or reduce the frequency of stent thrombosis following stenting for STEMI? Optimum stent deployment and optimum stent expansion has been shown to be critical in preventing ST with elective stenting, and we believe has the same importance with stenting for STEMI. Under-expansion of stents may be more frequent with STEMI because thrombus and spasm may cause underestimation of vessel size. Thrombectomy has been shown to decrease the incidence of ST possibly by providing a better coronary lumen allowing fuller stent expansion.11 The liberal use of intra-coronary nitroglycerine, post-dilatation and Intravascular ultrasound (IVUS) guidance may all help to promote better stent expansion and may decrease the risk of subsequent ST. Secondly, the use of new generation antiplatelet agents can reduce the frequency of ST. ST was shown to be significantly reduced at one year with prasugrel and ticagrelor in the STEMI cohorts of the TRITON TIMI-38 Trial (1.6 versus 2.8 %, p=0.02) and the PLATO Trial (1.6 versus 2.4 %, p=0.03).4,5 Thirdly, ST may be reduced by using DES selectively. The HORIZONS-AMI trial found that only patients at high risk for TLR with BMS (insulin dependent diabetics, patients with small reference vessel diameter (<3.0 mm) and long lesions [>30 mm]) benefited with reduced TVR with DES (see Figure 4).12 Patients with low and possibly intermediate risk for TLR and patients with poor compliance may best be treated with BMS rather than DES. Finally, the use of new generation DES can greatly decrease the frequency of ST. Two-year outcomes with everolimus-eluting stents (EES) compared with PES in ACS patients in the Clinical evaluation of the XIENCE V everolimus eluting coronary stent system (SPIRIT) and Everolimus-eluting stents and paclitaxel-eluting stents for coronary revascularization in daily practice (COMPARE) trials found a lower frequency of MACE (8.7 versus 11.0 %, p=0.04) and ST (1.3 versus 3.4 %).13 With these techniques – optimal stent deployment, use of new-generation antiplatelet agents, selective use of DES and use of new generation DES – the frequency of ST following stenting for STEMI should be greatly reduced.
Management of Coronary Thrombus
Management of a large thrombus burden (LTB) in the infarct-related artery at the time of primary PCI can be a difficult challenge for the interventionalist. A LTB has been associated with worse procedural outcomes with more distal emboli and no-reflow and poorer microvascular reperfusion as evidence by lower frequencies of angiographic myocardial blush grade 3 (MBG3) and complete electrocardiographic ST-segment resolution.11 LTB has also been associated with increased two-year MACE.11 Options for management of thrombus include pharmacological therapy (generally GPI) and thrombectomy devices.
Aspiration thrombectomy came into frequent use following the publication of the Thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction (TAPAS) trial.14 In this trial, the use of aspiration thrombectomy was associated with a higher frequency of MBG3 and complete (>70 %) ST-segment resolution and at one year was associated with lower mortality (4.3 versus 8.0 %, p=0.04).15 The mortality reduction was surprising and may have occurred by chance, since the study was not powered for mortality outcomes and a 46 % reduction in mortality does not seem reasonable. Nevertheless, based on the TAPAS trial and a number of smaller trials and meta-analyses, aspiration thrombectomy has received a Class IIa indication with primary PCI in the American College of Cardiology (ACC)/American Heart Association (AHA) 2009 and European Society of Cardiology (ESC) 2010 STEMI Guidelines.
The use of mechanical thrombectomy with primary PCI has been more controversial. The Angiojet in acute myocardial infarction (AiMI) trial evaluated rheolytic thrombectomy in 480 high-risk STEMI patients and found no benefit and possible harm.16 The primary endpoint, infarct size by sestamibi scans, was larger with rheolytic thrombectomy (12.5 versus 9.8 %, p=0.02) and mortality was higher (4.6 versus 0.8 %, p=0.02). There are several possible explanations for these disappointing results. The design of the trial meant that randomisation occurred after angiography and patients with LTB may have been excluded because the operators thought that thrombectomy would be beneficial. Also, patients without visible thrombus, who are unlikely to benefit from thrombectomy, were included. In this trial, pre-dilatation was frequently performed and the thrombectomy device was generally not activated until the lesion had been crossed. Both of these techniques could predispose to distal emboli. There was also an imbalance in baseline variables with the control group having a higher incidence of baseline TIMI 3 flow. And mortality was unusually low in the control group. The AngioJet rheolytic thrombectomy before direct infarct artery stenting with direct stenting alone in patients with acute myocardial infarction (JETSTENT) trial recently evaluated rheolytic thrombectomy with primary PCI and was designed to avoid some of the problems in AiMI.17 Only patients with a grade 3–5 thrombus were included, predilatation was avoided and the device was activated in an antegrade fashion. In this trial, rheolytic thrombectomy was associated a higher frequency of ST-segment resolution (85.8 versus 78.8 %, p=0.043) but no significant reduction in infarct size (11.8 versus 12.8 %, p=0.40). At one year, MACE was lower with thrombectomy (14.9 versus 22.7 %, p=0.036). Although this trial did not meet its primary endpoint, the results of this trial support the safety and possible efficacy of rheolytic thrombectomy in patients with a grade 3–5 thrombus.
Based on these trials and these data, a reasonable approach to the management of coronary thrombus with primary PCI can be formulated. Patients with little or no thrombus probably do not need thrombectomy. Patients with moderate to large thrombus (grade 3–4 thrombus i.e. thrombus size ½ to >2 times the vessel diameter) should be treated with aspiration thrombectomy. In patients with giant thrombus or stent thrombosis, rheolytic thrombectomy probably offers the best approach and should be combined with GPI. Giant thrombus is not very well managed with aspiration thrombectomy, which usually results in incomplete thrombus removal and often results in distal emboli.
Recently, the use of intra-coronary GPI has been used as a strategy for the management of coronary thrombus. This strategy has recently been evaluated in the Infuse anterior myocardial infarction (INFUSE-AMI) trial which has a 2x2 randomisation to aspiration thrombectomy, intra-coronary abciximab through an infusion catheter, neither strategy or both strategies.18 The primary endpoint of this trial, infarct size measured by MRI at 30 days, was reduced significantly, but only modestly, by intra-coronary GPI and was not affected by aspiration thrombectomy.
Optimum Reperfusion Strategies in ST-elevation Myocardial Infarction Patients Presenting to Non-percutaneous Coronary Intervention Hospitals
The management of reperfusion therapy in patients presenting to non-PCI hospitals with moderate to long delays to primary PCI has been very controversial. The 2007 ACC/AHA STEMI guidelines stated that STEMI patients presenting to non-PCI hospitals who cannot be transferred and undergo primary PCI within 90 minutes of first medical contact should be treated with fibrinolytic therapy. The new updated 2011 ACC/AHA guidelines and the ESC guidelines extend that time limit to 120 minutes ‘as a system goal’. Currently, our options for reperfusion therapy for patients presenting to non-PCI hospitals include: transfer for primary PCI, local fibrinolytic therapy or a combined pharmacological and mechanical reperfusion strategy which includes facilitated PCI and the recently described pharmaco-invasive strategy.
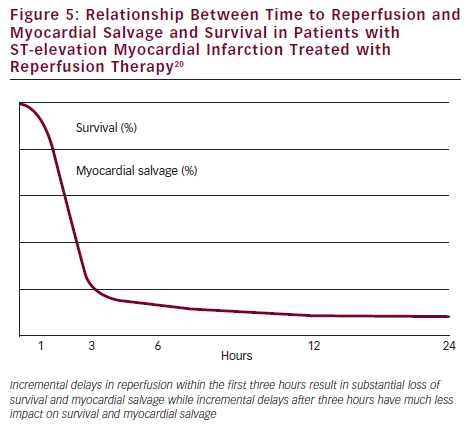
Facilitated PCI is a strategy in which pharmacological therapy (usually fibrinolytic therapy or half dose fibrinolytic therapy plus GPI) is given as soon as possible after the onset of STEMI to establish early reperfusion followed by transfer to a PCI hospital for planned emergent PCI. Several trials have evaluated this approach and the largest is the Facilitated intervention with enhanced reperfusion speed to stop events (FINESSE) trial, which randomised over 2,400 STEMI patients to one of three strategies: transfer for primary PCI with abciximab, up front abciximab followed by transfer for facilitated PCI, or half dose reteplase plus abciximab followed by transfer for facilitated PCI.19 TIMI 2–3 flow on initial angiography was more frequent with both facilitated PCI strategies, but there were no differences in the primary endpoint of death, shock or congestive heart failure (CHF) at 90 days and there was more bleeding with facilitated PCI. Why did facilitated PCI not work? The answer appears to be related to the fact that incremental reperfusion delays impact survival and myocardial salvage dramatically in the first three hours after the onset of infarction but have much less effect after three hours (see Figure 5).20 In patients who present in the first 60–90 minutes, delays to reperfusion are critical, because incremental delays have a major impact on outcomes. In patients who present later (after 90–120 minutes) delays are much less important, because there are little differences in outcomes with incremental delays beyond three hours. Consequently, the early reperfusion advantage offered by facilitated PCI benefits only a very small minority of STEMI patients presenting to non-PCI hospitals – those patients who present very early and are at high clinical risk.21,22 This group comprises about 16 % of the STEMI population.
The recently studied pharmaco-invasive strategy for reperfusion offers another approach. This strategy gives pharmacological therapy (fibrinolytic therapy or half dose fibrinolytic therapy plus GPI) at a non–PCI hospital followed by transfer to a PCI hospital for urgent PCI. This is very similar to facilitated PCI but has been compared with fibrinolytic therapy given at the non-PCI hospital rather than transfer for primary PCI. Also, with the pharmaco-invasive strategy, PCI is performed urgently at six to 24 hours rather than emergently as is done with facilitated PCI. This strategy has been evaluated in the Combined abciximab reteplase stent study in acute myocardial infarction (CARESS) and Trial of routine angioplasty and stenting after fibrinolysis to enhance reperfusion in acute myocardial infarction (TRANSFER-AMI) trials both of which found a reduction in the primary endpoint of death, reinfarction or recurrent ischaemia with the pharmaco-invasive strategy.23,24 Based on the results of these trials, this strategy has been given a Class IIa indication for patients treated with fibrinolytic therapy at non-PCI hospitals by the ACC/AHA and ESC guideline committees.
If we look at our options for reperfusion therapy for STEMI patients presenting at non-PCI hospitals, transfer for primary PCI has been shown to be better than facilitated PCI even with long delays to PCI and a pharmaco-invasive strategy, which is similar to facilitated PCI, has been shown to be better than fibrinolysis alone. So the optimum reperfusion strategy for STEMI patients presenting at non-PCI hospitals appears to be transfer for primary PCI even with delays of 120 minutes and longer except for a small group of patients who present very early (<60 minutes), are at high clinical risk and low bleeding risk and have long delays to PCI. These patients can be best managed with a pharmaco-invasive strategy.







