Mitral regurgitation (MR) is the most common valvular heart disease (VHD), and it is associated with significant mortality and morbidity.1 It affects around 10% of the population, and its incidence is increasing, making it the most prevalent valvulopathy in people over 75 years of age in the US and the second most frequent in Europe.2 MR can present as either primary or secondary.
Primary MR, also termed degenerative MR (DMR), encompasses leaflet and subvalvular apparatus pathology associated with various aetiologies, such as prolapse syndrome, flail leaflet, rheumatic heart disease, infectious/inflammatory state, radiotherapy and specific drugs, including certain anorectic medications, along with collagen vascular disease.3,4
Secondary MR, also known as functional MR (FMR), occurs when the annulus expands because of left ventricular (LV) remodelling and dilation. The cause can be ischaemic, non-ischaemic or atrial. Ventricular FMR is typically caused by annular dilatation in non-ischemic dilated cardiomyopathy. Ischaemic FMR occurs as a result of leaflet motion restriction caused by regional LV wall dysfunction and remodelling. Atrial FMR is increasingly being recognised and seen in patients with AF, hypertension, and a preserved ejection fraction.5 Standardised echocardiographic measures for identifying severe DMR and FMR include a vena contracta ≥7 mm, a regurgitant fraction ≥50%, an effective regurgitant orifice area of ≥40 mm2 and a regurgitant volume of ≥60 ml (or ≥45 ml for low-flow situations such as FMR).3 It is imperative for successful treatment and outcomes to identify aetiology and quantify degree of MR.
Mitral Regurgitation Treatment
Mitral valve (MV) disease management encompasses comprehensive imaging and case-by-case evaluation by the Heart Team, in which the experience of the team is key.
Individuals with chronic severe FMR (stage C or D), according to the American College of Cardiology/American Heart Association (ACC/AHA) VHD recommendations, should initially receive guideline-directed medical therapy (GDMT) to optimise primary LV dysfunction. In a patient with FMR due to LV systolic dysfunction and continuing severe symptoms despite GDMT, the recommendations suggest mitral transcatheter edge-to-edge repair (M-TEER) for those who have suitable anatomy and haemodynamics. Surgery may help improve symptoms in certain individuals with severe FMR. MV replacement is preferable over repair.6
The European Society of Cardiology (ESC) guidelines propose transcatheter edge-to-edge repair (TEER; class 2a, level of evidence [LoE] b) for selected patients who meet the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) criteria, as well as M-TEER for patients who do not meet the COAPT criteria but may benefit from improved symptoms and quality of life (QoL; class 2b, LoE c). Patients with chronic FMR who do not meet the COAPT criteria include those with advanced heart failure (HF) and drastically reduced LV ejection fraction (LVEF <20%), as well as those with atrial FMR but intact LVEF, cardiogenic shock, inotropic support, LV end-systolic dimension >7 cm, home oxygen use, or severe tricuspid regurgitation.4,7,8
Emerging research suggests that M-TEER promotes atrial reversal remodelling and improves symptoms.9 In the European Registry of Transcatheter Repair for Secondary Mitral Regurgitation, the prevalence of atrial FMR was 7.8%, and the results were encouraging in patients regarded as high risk for surgery, particularly with advanced HF symptoms.10
Furthermore, it is critical to consider the challenges to successful GDMT optimisation, as well as to understand the advantages of high-intensity treatment over traditional GDMT sequencing. GDMT optimisation, combined with CRT if necessary, might take months with the standard approach. Evidence shows that rapid GDMT commencement and up-titration outperform a gradual strategy.11,12 As demonstrated in STRONG-HF, rapid commencement and the increasing of titration can save lives and reduce hospitalisation.12
In terms of DMR, ideally medical therapy should be complemented with early MV repair targeting the underlying pathology of MV mechanical incompetence. Surgical MV repair carries less than 1% mortality in more than two-thirds of non-emergency cases.13 Despite its safety and efficacy, many patients with severe MR go untreated owing to the possibility of surgical complications. According to some studies, less than half of DMR patients underwent surgery, whereas more than 30% did not meet the criteria, were considered high surgical risk, or rejected treatment.14 The Heart Team may consider M-TEER in patients with high or prohibitive surgical risk (class 2b, LoE b) based on the randomised EVEREST II study and numerous registry outcomes.15–17 According to the ACC/AHA guidelines, M-TEER is a class 2a recommendation for selected patients in New York Heart Association (NYHA) class III or IV.6
Another growing area of M-TEER consideration involves cardiogenic shock due to acute MR or acutely decompensated known FMR. Small observational studies suggest that for MR patients with significant LV failure and cardiogenic shock, TEER can be used as a bridging therapy.18,19 Patients with COAPT-like versus COAPT-ineligible criteria after M-TEER had similar procedural success rates and QoL improvement, although COAPT-ineligible patients had a poorer 1-year survival rate.20 M-TEER has been used successfully as an alternative to surgical intervention in high-risk patients with acute MR caused by papillary muscle rupture following MI.21,22 M-TEER has been linked to decreased in-hospital and 1-year mortality rates compared with surgical MV repair or replacement.23 Thus, for DMR and FMR patients there is a growing need for additional types of MV intervention. M-TEER offers a solution for this high-risk MR patient population. Procedural planning includes detailed characterisation of MV anatomy such as leaflets, annulus and subchordal apparatus, using transoesophageal echocardiogram (TEE). The ACC transcatheter valve certification programme can also help identify appropriate referral centres that use a multidisciplinary heart valve team with a shared decision-making model and patient-centred approach.6
Mitral Transseptal Transcatheter Devices
MitraClip Device
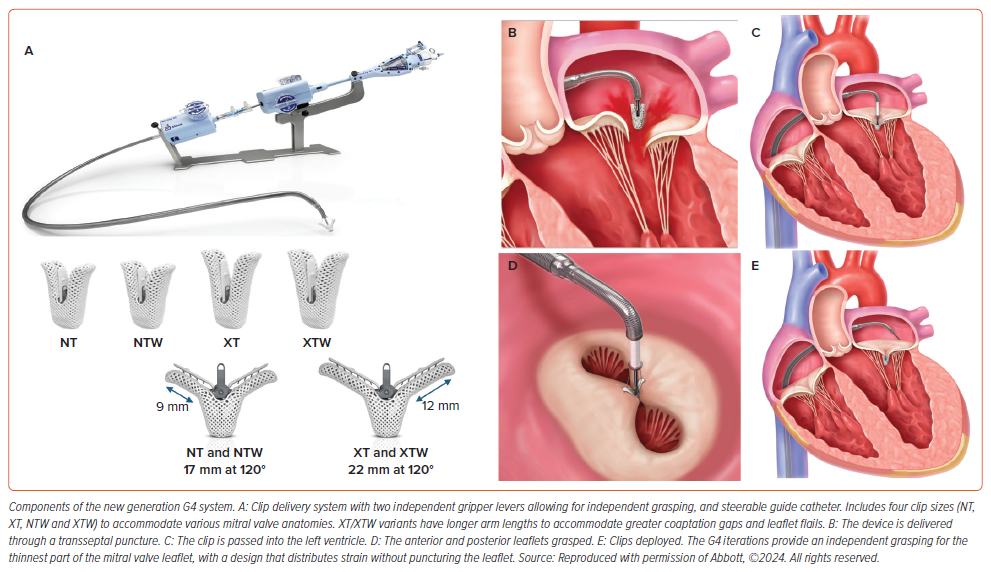
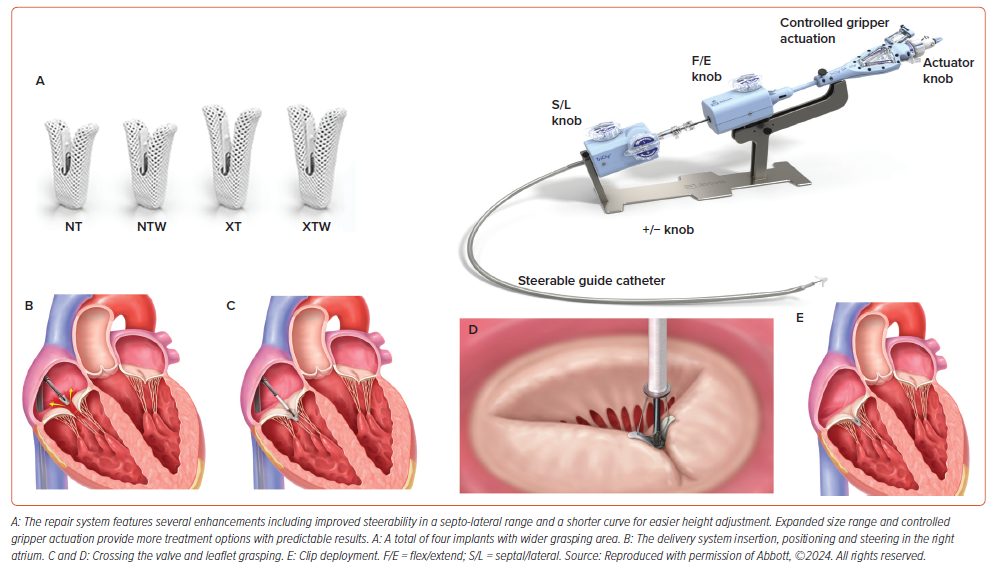
The MitraClip device (Abbott) consists of two essential elements: a steerable guide catheter and a clip delivery system featuring a detachable clip (Figure 1). This configuration enables precise manipulation of the clip along multiple axes, thereby improving the efficacy of M-TEER. The fourth-generation MitraClip is available in four implant sizes (Figure 1) leading to reduced procedure duration and clip usage.24 The M-TEER procedure is now more suitable to a broader spectrum of patients, owing to enhanced accuracy and predictability in delivery, independent leaflet grasping, clip retrievability, broader and longer clip sizes, and advancements in adjunctive imaging capabilities.25
PASCAL System
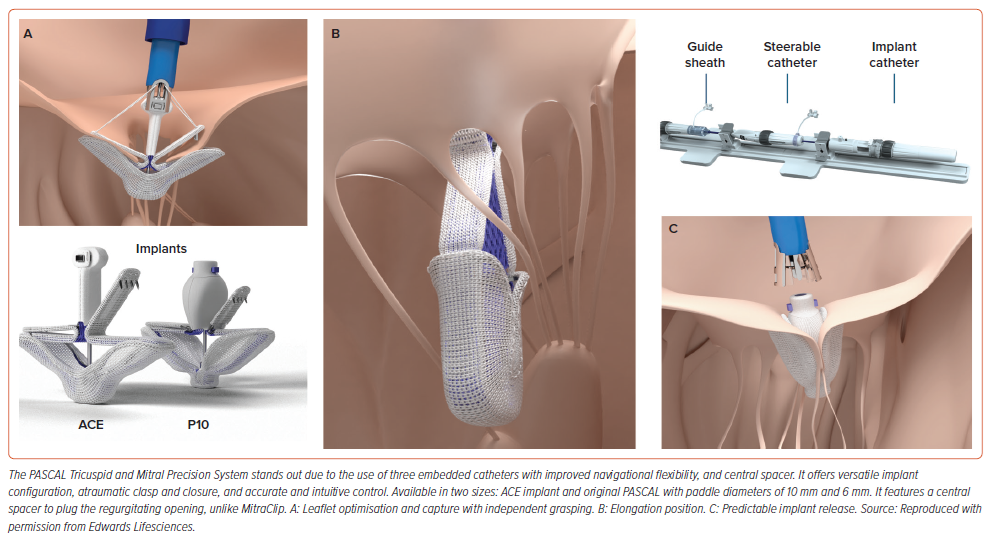
The PASCAL implant system (Edwards Lifesciences) consists of three embedded catheters with two nitinol-based implant sizes. It enables independent leaflet grasping, allowing for leaflet optimisation, as well as a staged capturing strategy with the centre spacer to cover parts of the coaptation gap in the main MR jet area, potentially reducing stresses on the MV leaflets (Figure 2).The CLASP IID trial showed that the PASCAL device was not inferior to MitraClip in the treatment of DMR.26 Although early research also suggests efficacy in patients with FMR, this is now being studied further in the US in the ongoing CLASP-IIF trial (NCT03706833).
NeoChord NeXuS System
Repairing the chordal system can be fundamental in the reduction of anatomical prolapse and optimisation of annular plane. Various surgical techniques have been successfully developed and used over the years. However, transseptal transcatheter techniques are currently in the early phase. The first-in-human study in 2022 demonstrated the feasibility of the NeoChord NeXuS system (NeoChord) to restore physiological mitral leaflet function by transseptal transcatheter implantation.27 Implementing transcatheter options may reduce the need for surgery, but it will not eliminate the need for surgery or other treatments. However, some disadvantages include a lack of tactile evaluation of tissue quality and determining where the chord was placed on the leaflet with imaging. Anatomical problems include the inability to repair a chord while the heart is beating.
M-TEER Considerations
M-TEER should be preceded by a thorough clinical evaluation, as well as an understanding of the risks and benefits, and the recognition of anatomical barriers.
Clinical approaches to determine medical futility versus benefit in some of the most severely ill patients may be difficult. For instance, FMR is more than simply a valve illness in these individuals; we may need to understand where they are on the disease spectrum (stage I LV involvement, stage II left atrium involvement, stage III pressure volume overload, and stage IV biventricular failure) for better post-interventional survival and symptomatic improvement.28
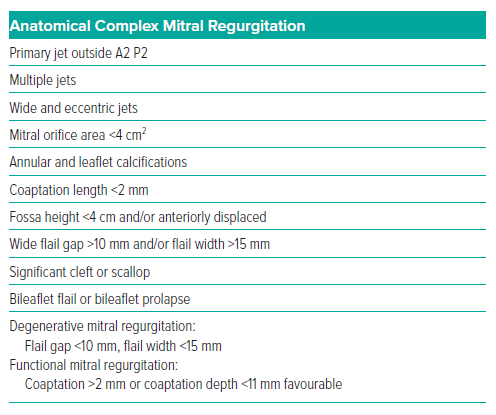
Furthermore, anatomical complexity may range from ideal to impossible for TEER. When choosing a device, anatomical features to consider include mobile leaflet length, gap zone, MV area, thin leaflets, commissural jets and presence of Barlow’s disease.29 The M-TEER technique relies heavily on a thorough evaluation of anatomical complexity (Table 1).
The posterior leaflet length, encompassing around two-thirds of the annular circumference, has a considerable influence on device selection and procedural subtleties. The ideal posterior leaflet length exceeds 10 mm, whereas a length less than 6 mm provides technical obstacles. Assessment of leaflet calcification degree is critical, especially in the gripping area for M-TEER, where uncalcified portions are preferable.29,30
Devices with extended arms (e.g. XT, XTW or PASCAL) are more effective at reducing MR, particularly when numerous implants are used.31 In short and/or thin tethered posterior leaflet as seen in FMR patients, devices with extended arms (e.g. XT, XTW) should be avoided to prevent single leaflet device attachment (SLDA) or leaflet injury. The PASCAL devices appear to be more favourable in these situations due to a flexible nitinol design and horizontal orientation of the grasping elements.29
Severe calcifications in the mitral annulus may restrict the MV area and prevent the annular return to its natural shape following TEER.29,30 The implantation of a PASCAL P10 has been demonstrated to lower MV area by 47%, whereas MitraClip NTR and XTR implants reduced it by 52% and 57%, respectively. The maximum reduction will occur in the A2/P2 position, with modest reduction in commissural placement.32 MV apparatus pathology can increase the risk of device entrapment during TEER. Adequate chordal support significantly promotes effective M-TEER outcomes, while insufficient support, as found in cases of papillary muscle failure after MI, can complicate the treatment.29,30 Implants with small arms (e.g. NT/NTW) are preferred for isolated commissural lesions and annular calcifications to prevent an increase in transmitral gradient following M-TEER.33,34
Anatomically difficult cases of MR may not be repairable due to poor reduction, stenosis or procedural challenges. Recent trials have shown that M-TEER can treat complex anatomy with reasonable success.26,35
It is vital to consider the importance of procedural clinical consequences, not only anatomical ones, given that non-ideal outcomes may nonetheless lead to better QoL in high-risk patients.
M-TEER Outcomes
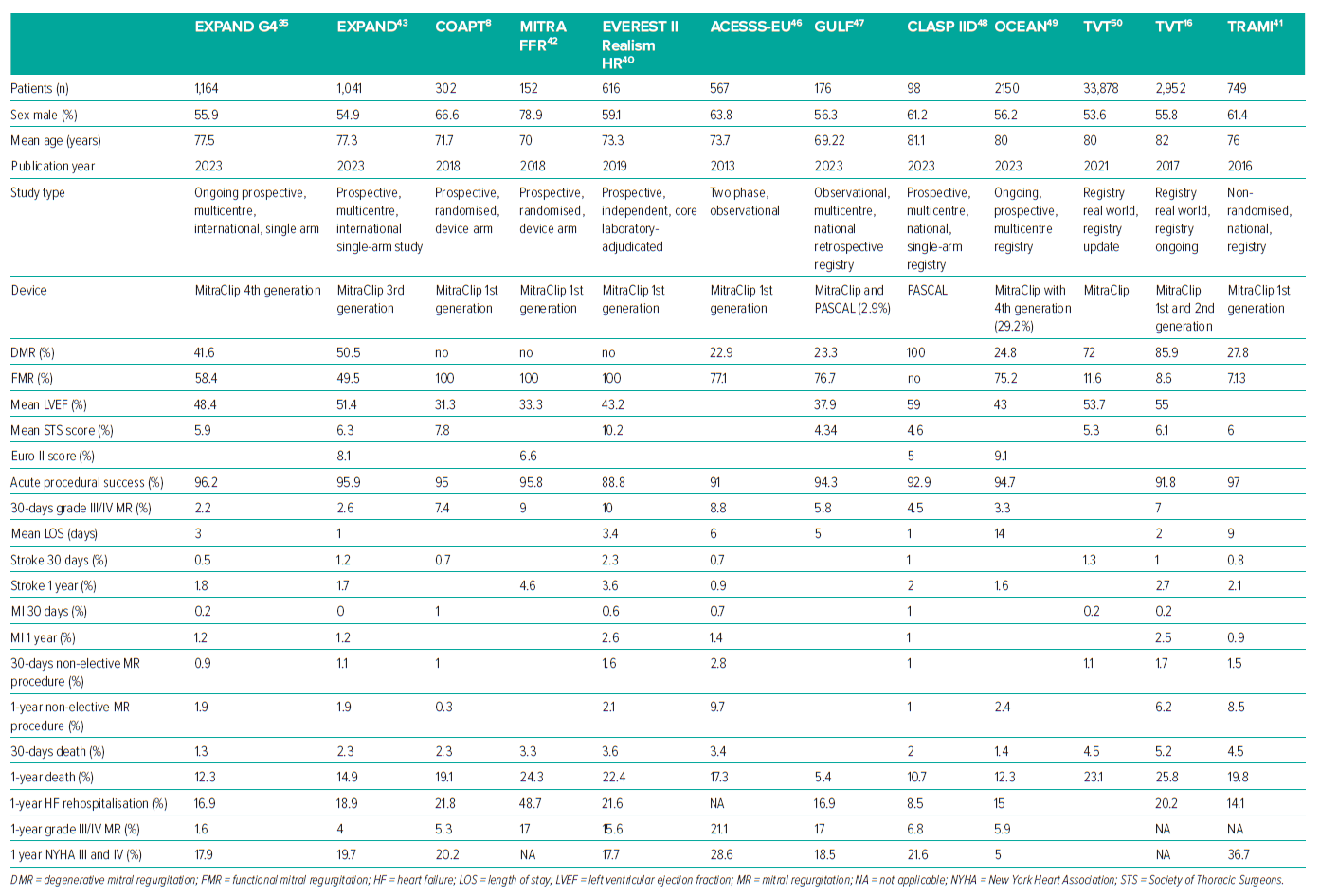
The initial MitraClip was implanted 20 years ago and has since been used in more than 150,000 patients worldwide. M-TEER has been demonstrated to improve symptoms, decrease MR by 2–3 grades, and stimulate reverse cardiac remodelling in patients with DMR that is considered too risky for surgery.15,16 Chronic FMR has a poor prognosis, whether from ischaemia or non-ischaemic causes.36,37 Several randomised controlled trials and retrospective registries, including early EVEREST Phase I and Cohort, have shown promising outcomes with low complication rates and a significant decrease in major adverse event (MAE) rate since 2005, considering both devices (Table 2).17,38 The clinical outcomes considered in this review include procedural success rates, all-cause mortality, HF hospitalisation, NYHA functional classification and QoL as measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score.
Procedural Success
The success of a TEER operation can be described as a reduction in the MR to no more than mild or, in situations with challenging anatomy, MR reduction by at least one grade from the baseline and no more than moderate (2+) in severity.39 According to the most recent data, implantation and post-procedural success rates are as high as 98.0% and 96.2%, with an average of 1.4 clips inserted per patient.35 TEER initially had more stringent inclusion criteria, as evidenced in the early EVEREST trials, but with the development of experienced centres, cases involving more difficult anatomies have been treated successfully.17,26,35,38
All-cause Mortality and Major Adverse Events
Low all-cause death rates were observed at 30 days and maintained for 1 year in multiple studies and registries (Table 2). The most recent study showed a 30-day mortality rate as low as 1.3%.35
Previous studies reported relatively high 1-year all-cause death rates, with 22.8% in the EVEREST II-REALISM high-risk study investigation, 20.3% in TRAMI, 24.3% in MITRA-FR and 19.1% in COAPT.8,40–42 In EXPAND’s studies using fourth- and third-generation M-TEER devices, 1-year all-cause mortality decreased to 12.3% and 14.9%, respectively.35,43
The 2-year mortality outcomes of the COAPT trial demonstrated reductions in mortality, with a number needed to treat of 6 to prevent one death.8 In propensity-matched individuals who received MV repair rather than replacement, 4-year mortality was 3.5% versus 12.1%.44
The 5-year follow-up outcomes of the COAPT trial were positive, with significant improvements in MV disease-related mortality. Death from any cause occurred in 57.3% of the device group and in 67.2% of the control group in a 5-year period (HR 0.72; 95% CI [0.58–0.89]).45
Low MAE rates were also observed, including low MI, with stroke rates at 30 days and sustained to 1 year (Table 2).16,41,46–50 The MAE rate for M-TEER has lowered from 15% in 2005 to less than 3.5% in 2020, despite recent advances in the treatment of complex lesions.15,51,52
Several factors, including advances in HF pharmacotherapy and more standardised patient selection, may contribute to improved outcomes. Furthermore, the decrease in 1-year all-cause mortality in DMR and FMR patients may be attributed to improved MR reduction as a result of advancements in M-TEER treatment, higher operator expertise, and superior imaging.
Quality of Life
Several studies and worldwide registries have found considerable increases in functional class, QoL and hospitalisation rates (Table 2).
Recent studies have shown that freedom from HF hospitalisations might be as high as 91.5%, with NYHA III/IV functional class ranging from 17.9% to 21.6% after 1 year.35,48 Significant QoL improvements of 18.5 points in KCCQ score at 1 year from baseline have been noted, with EVEREST II showing a significant increase in the 36-item Short Form Health Survey (SF-36) physical and mental QoL ratings from baseline to 12 months (n=191, p<0.0001).35,53 These findings add to the evidence that patient-oriented outcomes in MR patients are stable at 1 year following M-TEER therapy.
Predictors of Outcomes after M-TEER
Clinical, imaging and procedural variables can all be linked to negative outcomes after TEER, both immediately and later on.
Severe HF, chronic renal illness, male sex, tricuspid regurgitation and baseline pulmonary hypertension, anaemia-related blood transfusions, stroke, endocarditis, pulmonary embolism and pericardial effusion are all risk factors for in-hospital mortality.54–56
Strong clinical predictors of long-term all-cause mortality include ischaemic cardiomyopathy prior valve interventions, AF, renal impairment, surgical risk score and NYHA functional class.41,57,58 Prior valve procedures, such as a history of aortic valve surgery, independently predict longer-term mortality (1-year and 2-year mortality), after M-TEER.41,59
Increasing logistics EuroSCORE predicts mortality in patients with ischaemic MR, but not in those with non-ischaemic aetiology.60 A Society of Thoracic Surgeons (STS) score ≥12 was demonstrated to correlate independently with all-cause mortality following M-TEER.61 NYHA functional class IV at baseline predicted 1-year mortality in an independent manner.8,41 Lower baseline KCCQ scores were associated with HF hospitalisations in both the GDMT and intervention groups.8 Such findings indicate a strong link between low QoL (as measured by KCCQ) and negative outcomes.
Baseline echocardiographic parameters, such as LV function, pulmonary artery pressure, LV dimension, right ventricular (RV) function and the presence of tricuspid regurgitation, have a significant impact on all-cause mortality.59,62,63 Device failure attributed to operator error, conversion to surgery, clip placement failure, and residual severe MR are all significant predictors of increased all-cause mortality.41
M-TEER Complications
M-TEER has heralded a new era in the management of MR. However, despite its efficacy and advantages, this innovative technique is not without challenges and potential complications. From concerns such as device failure, access site bleeding and pericardial tamponade to the looming risks of thromboembolic events and other complexities, a thorough understanding of the intricacies surrounding M-TEER is crucial for both healthcare providers and patients navigating this advanced treatment modality. Potential complications can be categorised into device-related issues including thrombotic events, pericardial effusion, access site complications and late occurrences. Perioperative TEE imaging will ensure a careful evaluation of device integrity, position, stability and interaction with adjacent structures.64,65
Functional Device Failure
Persistent Mitral Regurgitation
Accurate post-procedure quantification of residual MR is crucial but challenging due to the transformation of the MV into a multi-orifice structure with multiple (often eccentric) residual jets.65 Early studies showed a reduction in MR to <2+ at discharge in less than 80% of cases (EVEREST I 64%, EVEREST II 77%). However, recent research involving third- and fourth-generation MitraClip devices has achieved MR reduction to <2+ in more than 95% of cases.17,24,38,43 Notably, 1-year MR ≥3+ was observed with the MitraClip-EXPAND G4 (1.6%), MitraClip-EXPAND G3 (4%) and PASCAL-CLASP II device (6.8%), highlighting continued improvements in M-TEER therapy.35,43,48 Persistent MR serves as a significant prognostic indicator for mortality and rehospitalisation during the post-implantation follow-up of patients who have undergone M-TEER.16,46,66 Procedural echocardiography for MR evaluation needs to be optimised, focusing on LV/LA pressure, haemodynamic conditions, asymmetric jets and anatomical challenges (Table 3). TEE will aid in the understanding of the underlying mechanisms of MR, to ensure optimal treatment.
Mitral Stenosis
The Mitral Working Group of the Valve Academic Research Consortium characterises post-procedural mitral stenosis as a mean transvalvular pressure gradient >5 mmHg.67 However, many studies provide insufficient or missing information about MV stenosis rates and definitions, and the impact of increased mitral valve gradient (MVG) on outcomes is debatable.68
An MVG pressure of more than 5 mmHg has been associated with unfavourable clinical and functional outcomes in patients with DMR but not in those with FMR.69,70
Yoon et al. found no significant influence (MVG >5 mmHg) on primary outcomes in DMR patients, and the COAPT study indicated that increased MVG was not connected with composite all-cause mortality or HF hospitalisation in FMR patients.71,72 Further investigation is needed to determine the long-term prognosis in patients with elevated MVG after DMR and FMR TEER. Post M-TEER significant mitral stenosis may necessitate surgery. Clip placement should be avoided for patients with a high MVG (>5 mmHg) and an MV area <4 cm2.66,73
Structural Device Failure
Single Leaflet Device Attachment
SLDA can occur because of inadequate leaflet capture, poor tissue quality, excessive clip and leaflet tension, and chordal entanglement. SLDA is more prevalent in complex lesions, with decreasing rates over the years, ranging from 11% in EVEREST I to 1.7% in EXPAND G4, due to better implantation technique and device iterations.35,38
It can lead to MR recurrence or exacerbation, posing a serious adverse event risk. It can manifest acutely, subacutely (shortly after the procedure) or, rarely, late during follow-up. Insufficient leaflet grasping usually leads to SLDA, whereas SLDA post-adequate grasping is commonly due to leaflet tears or perforation.46,74 Enhanced intraprocedural imaging and guidance are crucial for optimal device position, grasping control and clip insertion, minimising SLDA risks.
In difficult cases, rapid pacing, adenosine, and independent grasping can aid in clip placement optimisation. If SLDA occurs, an additional clip can be applied, or surgery may be required. If nothing is done after the procedure, severe MR is known to increase mortality.
High hospital mortality (8.2%) and long-term mortality (median 163 days, 29.3%) are associated with implant failure due to SLDA.75
Clip Embolisation
During the implantation process, although very uncommon, a full clip detachment from both leaflets can occur, leading to embolisation. The prevalence of embolisation is less than 1%, with two studies showing rates of 0.7% and 0.1%.16,76 Surgical clip removal may be necessary if feasible. Clip embolisation may be linked to complex mitral anatomy and the implantation of multiple clips, particularly when the echocardiographic window is compromised by artefacts from other clips. Detailed intra-procedural imaging, ensuring a precise view of the device, leaflets, and sub valvular apparatus, is essential in minimising detachment risk.65,73
Isolated Leaflet Damage and Chordal Rupture
Securing an optimal clip position may involve multiple grasping manoeuvres, which might injure fragile leaflets. In rare cases, more than one clip may be required, introducing additional challenges with each subsequent clip deployment. Inserting a second or third clip is particularly difficult because the additional clip may unintentionally approach the LV, resulting in misalignment and significant damage such as leaflet tear or chord rupture.64 The available literature on this issue is minimal, with incidence ranging from 0% to 2%.24,36,68 To untangle, try using small movements, reverse steps, anterior and posterior torque, and device inversion/elongation. Leaflet perforation can be sealed using vascular plugs, but this may cause haemolysis. Addressing isolated leaflet damage poses challenges and surgery is often necessary in many cases due to the persistence of severe residual MR.73
Thromboembolic Events
The risk of thromboembolic events is increased during TEER, given that thrombus can propagate through the venous system and across the transseptal access, leading to ischaemic events and thromboembolism during device manipulation. TEER has been found to be associated with a low risk of complications such as MI, pulmonary embolism (PE) or stroke (Table 2).73 The occurrence rates for MI and PE typically range from 0% to 0.2%, while stroke happens slightly more frequently, ranging from 0% to 1.4 % in all cases.64,73
Factors such as thrombogenic material, chamber dilatation, low cardiac output and AF can increase the risk of device-related thrombus formation during medical procedures, however, incidence is low (<0.5%) in large registries.16,41 Furthermore, a diffusion MRI study found an 86% occurrence of new silent cerebral lesions after TEER in 27 patients, with cognitive decline linked to >3 lesions. Subsequent dementia risk exists due to silent cerebral embolism.77 Even while the prevalence of thromboembolic events is anecdotal and usually multifactorial, thrombus development in the delivery system may have disastrous repercussions. TEE will aid in the early detection of device-related thrombosis, while risk can be minimised by maintaining perioperative therapeutic anticoagulation with continual flushing of the delivery system. Perioperative risk stratification for underlying hypercoagulable conditions can also be addressed.
Transseptal Complications
Pericardial Effusion
A potential risk of inadvertent perforation of the pericardium through a misdirected transseptal puncture can lead to a pericardial effusion. Pericardial tamponade is now uncommon (<1%); however, pericardial effusion has previously been observed in 3.3% with a higher tamponade rate (1.9%).16,17,41,68 Greater incidence is anticipated in complex scenarios involving hypermobile septum, post-surgical septum, or in cases of chest wall deformities.64 TEE-guided optimal transseptal puncture should be performed at the mid-superior position (bicaval view) and posteriorly in the interatrial septum.
Persistent Septal Defects
Persistent atrial septal defects following TEER are frequently observed, with prevalence rates ranging from 40% to 50%.78,79 Prior investigations have yielded varying results: some indicate poorer clinical prognosis and a higher mortality rate in individuals with significant defects, whereas others suggest minimal long-term clinical repercussions.78,79
Bleeding Complications
Access Complications
The most common method for venous access in TEER is through the right femoral approach using a 24 F guiding sheath. However, the proximity of the femoral vein to the artery can lead to severe vascular complications such as arteriovenous fistula, haematoma requiring transfusion, pseudoaneurysm, retroperitoneal haemorrhage, infection, vessel rupture/perforation and thrombosis.80 Major vascular complication rates range from 1.4% to 4% and minor complications have similar rates, with no significant changes over time.16,41,68,80 Preventive measures, such as ultrasound guidance for vascular access, may minimise risk for access complications.
Bleeding
There is considerable overlap between MR and other cardiac comorbidities, such as AF. As a result, these patients may already be on oral anticoagulant or antiplatelet medication, and intraprocedural heparin raises the risk of bleeding even more.64,79 Severe bleeding requiring a blood transfusion has been documented in the range of 0–17%, and it is considered an independent predictor of in-hospital mortality.17,24,37,41,55,79–81 Interestingly, less than half of the bleeding incidents are linked to the access site. A significant proportion of bleeding originates from gastrointestinal sources, followed by urinary tract, pericardial effusion, lines, or have obscure origins.79,81 Newer devices are reducing the possibility of bleeding events; however, a standardised approach is required to balance the risk of bleeding incidents with that of thromboembolic events.
Late Events
Endocarditis of the MV following TEER is uncommon, with reported rates of 0–2.6%.82,83 Most M-TEER endocarditis events occur in the first year (75%), resulting in surgical valve replacement in approximately 70% of cases and a 40% in-hospital death rate.82 Rare cases of chronic haemolytic anaemia, post-cardiac damage syndrome and interatrial septal dissection have also been documented following M-TEER.84–87
M-TEER Conclusion and Future Directions
Over the last two decades, M-TEER therapy has made substantial progress: from discovering that TEER can be done, as shown by the EVEREST MitraClip (2003–05), to the EVEREST II randomised controlled trial, which demonstrated safety while lowering MR with a 77% success rate.17,38 The EVEREST II High Risk Registry led to Food and Drug Administration (FDA) approval of MitraClip in high-risk DMR in 2013, and COAPT demonstrated a reduction in mortality, leading to FDA approval of MitraClip for FMR in 2019.8,53 CLASP II D resulted in FDA approval of a second device for DMR in 2023, which, along with EXPAND G4 (2023), demonstrated outstanding outcomes in patients with complicated anatomy.35,48 Standardised patient selection is crucial, and experienced centres that tailor the procedure to the patient achieve excellent outcomes.
The PRIMARY study (NCT05051033) will either confirm TEER as a treatment for a broader population of primary MR patients or reinstate surgical repair as the gold standard of care. EVEREST II is a comparable trial, but was more than a decade ago and used the original MitraClip.53 Not every patient is a candidate for M-TEER, and significant progress has been made, with transcatheter MV replacement emerging as a feasible alternative, particularly with the transapical technique as well.
Further trials are required to assess the impact of improvements in technical design, efficiency and operator experience. Advanced VHD patients are often very ill, and GDMT may not be sufficient to enhance overall QoL; consequently, being able to deliver effective treatments for them despite the lack of considerable mortality improvement is an impressive accomplishment. We hope that in the future, this therapy will provide standardised care for a range of cardiology practice structures, reducing disparity in access to cardiac expertise across the world.
Tricuspid Regurgitation
The presence of any severity tricuspid regurgitation (TR) affects more than two-thirds of the population, with a prevalence of significant TR of 1.5% in men and 5.6% in women over the age of 70 in nearly one-third of patients undergoing left heart surgery, particularly MV surgery.88,89 Primary tricuspid valve (TV) dysfunction is due to a lack of leaflet coaptation due to intrinsic changes leading to excessive or restrictive leaflet mobility or perforation (e.g. carcinoid, endocarditis, trauma, radiotherapy, myxomatous disease, congenital disease). Secondary or functional TV disease is often related to right atrial (RA) or RV enlargement/dysfunction due to pulmonary hypertension, left-sided heart disease, AF or lead impingement from cardiac implantable electronic devices (CIEDs).90,91 Implantation of CIED RV leads provokes relevant TR in 20–30% of patients, which frequently progresses over time.92–95 Historically, more than 90% of patients with clinically relevant TR have not been offered a surgical approach, possibly due to the known high surgical mortality.96 There is a misconception that TR improves after treatment for left-sided heart disease, even though TR progresses in up to 25% of patients postoperatively.90,91
Tricuspid Regurgitation Treatment
Diuretics play a key role in managing the symptoms of TR.97 Surgical correction for TR is strongly emphasised in current guidelines, which classify TV surgery (class 1 LoE b-NR), particularly for patients with severe TR during left heart cardiac surgery (class 2a LoE b-NR).6,98–107 Similarly, the 2021 ESC/EACTS guidelines gave TV surgery a class 1 LoE c recommendation for symptomatic individuals with severe isolated primary TR and no severe RV dysfunction. Class 1 LoE b was given for patients with severe secondary TR undergoing left-sided valve surgery.4,101,103,108–110 Transcatheter interventions for TR are given a 2b LoE c recommendation only in symptomatic patients with severe secondary TR not suitable for surgery with expected QoL improvement.6 Isolated TV surgery has a mortality rate of ~10%, with better outcomes in specialised centres.100
TRI-SCORE predicts hospital mortality following the primary TR surgery and it showed good discrimination for in-hospital and 1-year mortality in patients undergoing transcatheter TV repair as well.111,112
Transcatheter TV interventions (TTVIs) are effective in selected patients, but long-term clinical benefits beyond improved QoL are yet to be demonstrated.
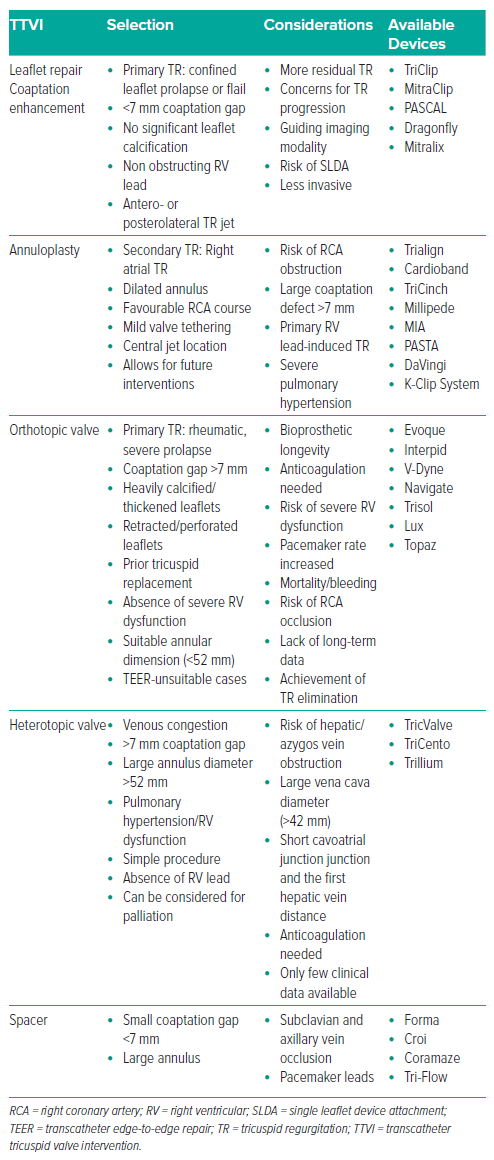
Palliation should be recommended when appropriate, and TTVIs should be considered for high-risk patients with a good prognosis. Multimodal imaging and right cardiac catheterisation are crucial for determining the mechanism and degree of TR. The choice of TTVI for primary TR is determined by parameters such as leaflet anatomy, annular diameter and regurgitation mechanism. Treatment choices for secondary TR are determined on whether the TR is atrial or ventricular, with RV function and RV–pulmonary artery coupling dictating the efficacy of therapy. Tricuspid interventional procedures are divided into four categories: leaflet modification/coaptation (T-TEER), annuloplasty, orthotopic transcatheter TV replacement and heterotopic caval valve implantation (CAVI; Table 3).
Currently, T-TEER is the most widely used technique when anatomically possible. The purpose of T-TEER is to improve leaflet coaptation and decrease TR. T-TEER should be reconsidered in individuals with unsuitable anatomy, including significant leaflet thickness, large and wide jets, short leaflets, and multiple coaptation planes. Transcatheter annuloplasty is one of the most anticipated therapies, offering long-lasting annular reduction, particularly in the secondary atrial TR population.113 Furthermore, orthotopic TTVI should be considered for patients with complex leaflet pathology caused by organic aetiology, while heterotopic CAVI has been used to protect organs from venous hypertension and minimise backflow-associated TR.114,115
T-TEER Devices
This review centres on T-TEER, which has become a feasible treatment option for patients with high-risk TR. There are now two T-TEER systems in use: the PASCAL and the TriClip (Abbott; a variant of the MitraClip), approved by the FDA in April 2024 based on data from TRILUMINATE Pivotal (Figures 2 and 3).116
TriClip use demonstrated a reduced 1-year death rate (7.1%) in the high-risk surgery group.116 The findings were supported by the real-world bRIGHT registry, which included more than 500 patients treated with the TriClip system.117 The PASCAL method was first used for the compassionate cohort in 2017.118 It had a low complication rate and a high survival rate, with significant and sustained improvements in TR, functional status and QoL after 1 year.119 The ongoing randomised CLASP II TR study (NCT04097145) will compare treatment outcomes using the PASCAL system to those with optimal medical therapy.119
T-TEER Outcomes
T-TEER is associated with high procedural success rates along with improvements in NYHA functional class, TR severity, QoL and patient-reported outcomes.120,121 The aetiology of TR is predominantly functional, with the most prevalent comorbidities being AF, hypertension, renal illness, diabetes and previous MI.4,122 Overall, in this T-TEER review, individuals had a high baseline fragility due to many coexisting comorbidities that posed a major surgical risk.
Procedural Success
In several studies the procedural success rate was high (98.8–100%), demonstrating excellent short-term outcomes.116,117,120,121,123 The success of the TriClip, MitraClip and PASCAL devices was at 97%, and they were associated with a significant reduction in TR volume, grading, tricuspid annular diameter, proximal isovelocity surface area radius and effective regurgitant orifice area at 30 days compared with baseline. However, there were no end differences in mean LVEF.124
In the bRIGHT post-approval study, 99% had successful implantation and 77% TR decreased to ≤ moderate grading after 30 days.117
The procedure duration ranges between 88 minutes and 153 minutes, with the total required length of hospitalisation being a median of 1–2 days.116,120,121,123 On average, a patient needed two clips.116,117,120 In previously reported experiences, the first clip was implanted anteroseptal in 91% of them.117,122,125 The availability of the XT clip improves procedural outcomes by making gripping easier, particularly in valves with high coaptation gaps. The NT clip was often left for greater commissural gripping when operators may expect a larger danger of entrapment with the sub-valvular equipment.121
In the TRILUMINATE Pivotal trial, durable repair was seen in 75% of subjects, with a sustained reduction in TR and stable device function when comparing 30-day and 2-year results. The TEER device (TriClip) mainly affected changes in TR measures rather than right heart remodelling measures.116,117,120–123 However, there was favourable remodelling of RV size and function in the setting of sustained reductions in TR despite the more variable dilatation of the tricuspid annulus and RA in this setting.116 Iatrogenic TV stenosis is seen in association with CIED leads due to valvular and/or subvalvular adhesions and in the long term following surgical TV replacement due to valve degeneration, but rarely after T-TEER.126 This stark difference provides safety assurance for the TEER procedure even when multiple clips are used. However, the question of how much post-TEER residual mean gradient is significant remains unknown. The current guidelines recommend a cut-off of ≥5 mmHg for the adjudication of significant TV stenosis.4
All-cause Mortality and Major Adverse Events
In medically treated patients with symptomatic TR, mortality ranges from 21% to 53% at 1-year follow-up.95,127,128 The TRI-REPAIR study showed a reduced all-cause mortality of 27% (8/30 patients) at 2 years and a cardiovascular mortality of 15.3%.113 Early data from the TRISCEND II trial demonstrated the effectiveness of the EVOQUE system (Edwards Lifesciences) – transcatheter TV replacement with a lower-than-expected MAE rate (27.4% versus 43.8%), effective TR elimination in more than 55% of cases with massive or torrential TR, and the remainder classified as severe TR.114
Moreover, the T-TEER studies had low rates of MAEs and mortality through 30 days, but there is no substantial reduction in death when compared with medical therapy alone beyond 1 year.116,117 The incidence of all-cause mortality was noted in TR patients post-TEER at a rate of 6%, similar to that of the cardiovascular mortality. The incidence of stroke (0%), MI (1%) and new-onset renal failure (5%) 30 days following TEER was largely insignificant.124 Despite the broad range of anatomies and coexisting conditions of the subjects treated in the real-world population, the MAE rate remained low through 30 days of follow-up. All-cause mortality occurred in only 1% of subjects at 30 days. Other clinical safety endpoints, including re-intervention (0.2%) and reoperation (0.4%) were also rare through 30-days follow-up, demonstrating the excellent safety profile of the T-TEER system.117 In TRILUMINATE Pivotal, the observed favourable early survivorship may have been related to the rigorous selection process and enrolment of patients with fewer overall coexisting conditions than in previous studies.116,129–131
Quality of Life
At 30 days after TEER, there was a substantial decrease in patients reporting NYHA class III–IV symptoms. Even with the diverse anatomies and progressed disease states treated in a real-world setting, clinical outcomes including average KCCQ score increase, and NYHA functional class were improved compared with previous studies of T-TEER despite the registries having more carefully selected cohorts.116,122,132 A KCCQ increase of ≥15 points was realised in 56.2% of subjects, which is increased from the 49.7% of subjects with at least a 15-point increase in the TEER group of the TRILUMINATE Pivotal trial. KCCQ score improvement was increased in subjects with residual TR of moderate or less at 30 days when compared with subjects with residual TR of severe or more.116,117
In single-arm studies, the mean QoL scores, including KCCQ, European Quality of Life–5 Dimensions (EQ-5D), SF-36 physical component and SF-36 mental component, demonstrated a statistically significant improvement after intervention.117,120,122,123
There was also a substantial rise in 6-minute walk test (6MWT) 1 year after the procedure compared with that before the TEE.124 6MWT improvements were also sustained over 24 months in the TRILUMINATE Pivotal trial, and the reduction in HF and general hospitalisation was more substantial at 2 years than previously reported, at 84% and 49%, respectively.116
Echocardiographic quantitative improvements following T-TEER are further enhanced by improvements in QoL; however, it is important to note that these are small patient samples that favoured individuals with a longer period of HF, as well as the assumption that HF hospitalisation did not vary with time.
T-TEER Complications
Single-arm observational studies have documented a range of complication rates, including significant bleeding (0–11.9%), SLDA (3.8–13%) and non-elective cardiovascular surgery due to the device (0.2%).117,120–123 A pooled analysis showed a 6% incidence of procedure-related major bleeding.124 Major bleeding is prognostically relevant in patients receiving transcatheter heart valve procedures, emphasising the need for improved techniques to avoid this complication.133
In the most recent trial, SLDA was subclinical and occurred in 7.0% of attempted TEER procedures within 30 days after the procedure. There were no new incidences up to 12 months. There were no cases of device embolisation or thrombosis in the TEER group. At the 30-day follow-up, eight patients in the TEER group had an average tricuspid gradient of ≥5 mmHg. There was no substantial difference in cardiac electronic rhythm device implantation within 1 year after the procedure.116 There were no reports of pulmonary thromboembolism, newly diagnosed liver failure, or embolism.122 There were no periprocedural deaths, conversions to surgery, device emboli, MIs or strokes.116,120
Predictors of Outcome after T-TEER
Procedural success with reduction in TR grade ≥1 on the transthoracic echocardiogram at the 30-day follow-up was the only predictor for reduced mortality and HF hospitalisation.122,123,125 With regard to residual moderate post-TTVI TR, in the TRILUMINATE Pivotal trial, ≤ moderate TR following T-TEER was associated with a nearly threefold decrease in 1-year mortality and HF hospitalisations compared with subjects with ≤ severe TR.116 Additionally, the TriValve registry reported that post-TEER TR ≥ moderate was associated with a 2–3-fold excess in all-cause mortality and HF hospitalisations at 1 year.134
TR can be caused by several different underlying conditions, and its reduction with TEER may not address the root cause of the VHD. Moreover, prognostication of TR patients should consider underlying RV dysfunction, right-sided HF and hepatic and renal involvement. Recent data suggest that patients with relevant concomitant TR and MR have a very high 1-year mortality of up to 33%, likely to be due to underlying biventricular failure.28
There is substantial variation in the reported 1-year all-cause mortality among different studies involving TTVIs. More longitudinal follow-up of survival and hospitalisations for HF will be important in determining the full potential and future of TEER.
T-TEER Conclusion and Future Directions
Despite ongoing research on transcatheter-based TV repair procedures, there is a growing body of evidence indicating positive outcomes. Even off-label use of the MitraClip device was associated with clinical QoL improvement, regardless of baseline RA pressure.135 More recently, the bRIGHT trial has shown an increased degree of comfort with regard to clipping around CIED leads, with 30% of patients having lead complication rates of less than 1%, which was even lower than those of lead extraction.117 The durable TR reduction, with few adverse events and significant clinical benefits seen thus far, are encouraging for transcatheter treatment of TR with transcatheter devices.
The problem in selecting the ideal time for valve repair stems from the fact that the severity of TR can differ based on the time of assessment of the same patient. The absence of a significant mortality and hospitalisation benefit could be attributed to TEER being performed too late in some patients, when the effects of chronic ventricular volume overload are irreversible. Furthermore, T-TEER may not be the best option for some patient populations to begin with, and other transcatheter platforms should be considered instead (Table 3).
The first FDA approval of two TTVI devices in 2024, TriClip for TV repair and the Evoque valve for TV replacement, will change the landscape for other devices as well. There has been remarkable progress with novel TTVI platforms involving T-TEER, annuloplasty, heterotopic and orthotopic valve replacement. Matching the patient to the suitable intervention early on is likely to be the best approach to improve outcomes, and one thing is certain: the TV is no longer the forgotten valve.
Gender Implications for Mitral and Tricuspid Transcatheter Interventions
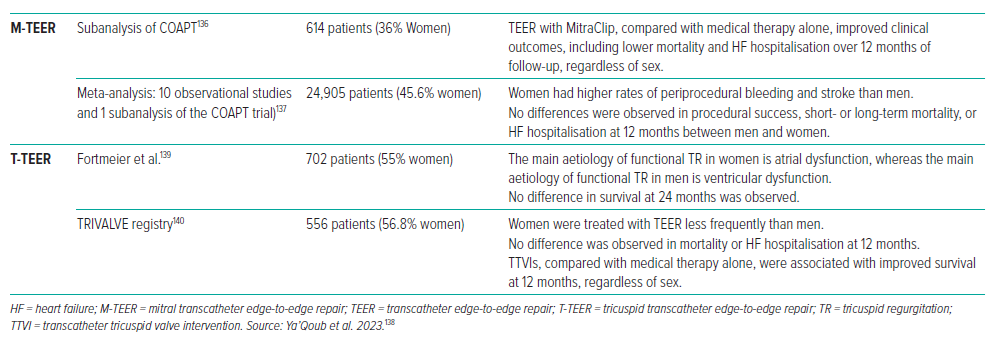
Patients having transcatheter therapy frequently exhibit differences due to a range of factors, including age at presentation, comorbidity prevalence, anatomical variances, and procedural outcomes between men and women. In M-TEER, women were older and had higher rates of bleeding and stroke. However, there was no significant difference in procedural success, heart HF hospitalisation or mortality (Table 4).136–138
The COAPT trial of M-TEER in secondary MR demonstrated a decrease in HF hospitalisations and mortality in patients with severe, symptomatic secondary MR despite GDMT.8
Significant TR is found to be more prevalent in women than men.88 Interestingly, in women, the primary aetiology of functional TR is atrial dysfunction, compared with ventricular dysfunction in men.139 Women are offered T-TEER less frequently than men.140 Regarding clinical prognosis, women tend to have a better prognosis than men in the overall population of patients with TR; however, there is no significant overall difference in mortality and HF hospitalisation between men and women treated with TEER.141,142 A targeted, unique patient strategy is necessary to minimise risks and problems whenever possible and indicated.
Conclusion
Significant advances have been made in transcatheter valve repair technologies, including imaging, a range of devices suitable for complex anatomies, and the operator’s overall experience. MR and TR are complex conditions, and as the number of interventional procedures grows, it is vital to choose the patient who will benefit the most from treatment. These devices have been designed to allow for the independent attachment of leaflets, wider and longer clasps for varied anatomies, and wider and narrower arm spans to reach or avoid other structures. Over time, TEER will surely adapt and evolve, complementing other evolving transcatheter modalities.
Similarly, the cost of TEER reduces over time, with lower device expenses and shorter hospital stays. As the ease of use, risk profile and cost improve, more facilities will opt to perform TEER, making it more accessible to more patients. The key to effective outcomes is early detection and referral, optimal imaging and a consistent multidisciplinary approach. Over the previous few years, TEER has developed into a safe, reproducible procedure with a positive effect on patients. Although future research will continue to define optimal usage, it will still have useful applications in a variety of subsets.