While the breadth of procedural offerings in interventional cardiology (IC) has exponentially expanded over the past four decades to include cardiac structural, peripheral arterial, and venous interventions, percutaneous coronary intervention (PCI) remains at the core of the field, accounting for the greatest percentage of therapeutic catheter-based procedures performed by IC practitioners in the US. Beginning with the historic series of coronary angioplasties performed by Dr Andreas Grüentzig in 1977, PCI has steadily advanced in its range of application and technical sophistication.1,2 Shortly after the landmark procedures were performed and reported at the Annual Scientific Sessions of the American Heart Association in 1977, a percutaneous transluminal coronary angioplasty (PTCA) registry was established at the National Heart, Lung and Blood Institute (NHLBI) in order to track the expansion, progress, and outcomes of this thenfledgling procedure.3,4 Dorros and colleagues reported on clinical outcomes and complications in the first 1,500 patients undergoing PTCA in the US (September 1977 to April 1981).5 The rate of PTCA success was 63 % at that time and the rate of major peri-procedural complications (myocardial infarction, emergency surgery, or in-hospital death) was 9.2 % with standalone mortality of 1.1 % (0.85 % in patients with single vessel disease; 1.9 % in those with multivessel disease).5 Even in the very earliest PTCA experience, lesion complexity and presenting acuity predictably affected clinical outcomes, a theme that has carried through to contemporary PCI.
Evolution of Complex Percutaneous Coronary Intervention
A recent publication from the NHLBI-sponsored PTCA and Dynamic registries sheds light on temporal trends in PCI spanning the several decades and multiple technological eras that have passed since the origins of the procedure. Specifically, the report documented the ingress of the field into clinical and procedural scenarios that fall under the rubric of complex PCI.6 Over the 20-year period studied, latter PCI cohorts were characterized by greater proportions of lesions bearing thrombus or calcium and patients with more medical comorbidities compared with the original PTCA cohort. Within the five consecutive Dynamic Registry waves studied (1997–2006), a period notable for the adoption of atherectomy, thrombectomy, cutting/scoring balloon angioplasty, and routine use of bare metal stents (BMS) and, later, drugeluting stents (DES), the proportion of American College of Cardiology/American Heart Association (ACC/AHA) Type C lesions intervened upon grew. Although initial technical success rates were reportedly high, lesions bearing markers of complexity, such as bifurcation disease, ostial location, calcification, and total occlusion, accounted for a significant proportion (9–36 %) of patients requiring repeat PCI within 30 days of their index intervention. Other investigators have independently confirmed in concurrent datasets that complex PCI (lesions evidencing thrombus, calcification, bifurcation or ostial location, chronic occlusion), was also associated with increased in-hospital and 1-year mortality rates compared with PCI of simpler lesions.7 Two large studies have now demonstrated that public reporting of PCI outcomes ostensibly influences the behavior and case selection choices of IC operators, suggesting that operators may be veering away from complex cases they believe will result in poorer outcomes.8,9 These data lend insight into the nuanced and, at times, conflicting considerations that factor into case selection and strategy for complex PCI. Fortunately, however, such considerations have not impeded the advancement of PCI techniques and technologies that have continued to flourish, fueled by scientific innovation and the clinical need for minimally invasive solutions to the growing burden of advanced coronary heart disease. Highlighted below are selected procedural and cost considerations in complex PCI subsets with particular focus on bifurcation disease, representing a commonly encountered, technically challenging, and well-studied complex lesion subset.
Landscape of Contemporary Percutaneous Coronary Intervention and Challenges Associated with Specific Lesion Sets
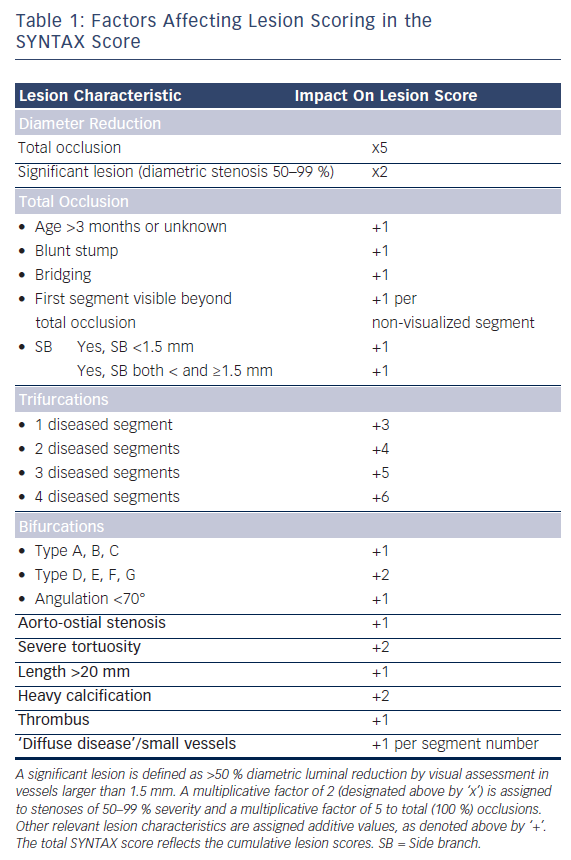
What began as simple balloon dilation of single, de novo coronary lesions has evolved into myriad variations on the theme of complex coronary intervention, the majority involving the implantation of one or more DES and a significant proportion utilizing adjunctive devices for PCI guidance and optimization. Indeed, 60 % or more of the DES used in the US are implanted in an ‘off-label’ capacity (in terms of US Food and Drug Administration [FDA] labeling), often in the context of the complex coronary lesions described below or for patients with significant medical comorbidities.10–12 It bears mention that while complex PCI subsets abound in clinical practice, a uniformly adopted definition for complex coronary artery disease (CAD) is lacking in the cardiovascular literature. Lesion scoring schema such as the prospectively validated SYNergy between PCI with TAXUS™ and Cardiac Surgery (SYNTAX) score provide valuable guidance for the decision to intervene and the strategy of percutaneous intervention.13 In the SYNTAX score (www.syntaxscore.com), which incorporates aspects of many pre-existing scoring systems, additive or multiplicative numerical values are assigned via a computerized algorithm to each obstructive lesion noted, based on dominance, number of lesions, segments involved per lesion, and six additional groups of queries relating to lesion characteristics (see Table 1).13 The total SYNTAX score represents the sum of the individual lesions scores and has prognostic value independent of medical comorbidity and other patient-specific metrics. In the SYNTAX trial, which randomly assigned 1,800 patients with multivessel or left main coronary artery (LMCA) disease to coronary artery bypass graft (CABG) surgery versus PCI with DES, higher scores portended poorer outcomes with multivessel PCI.13–15
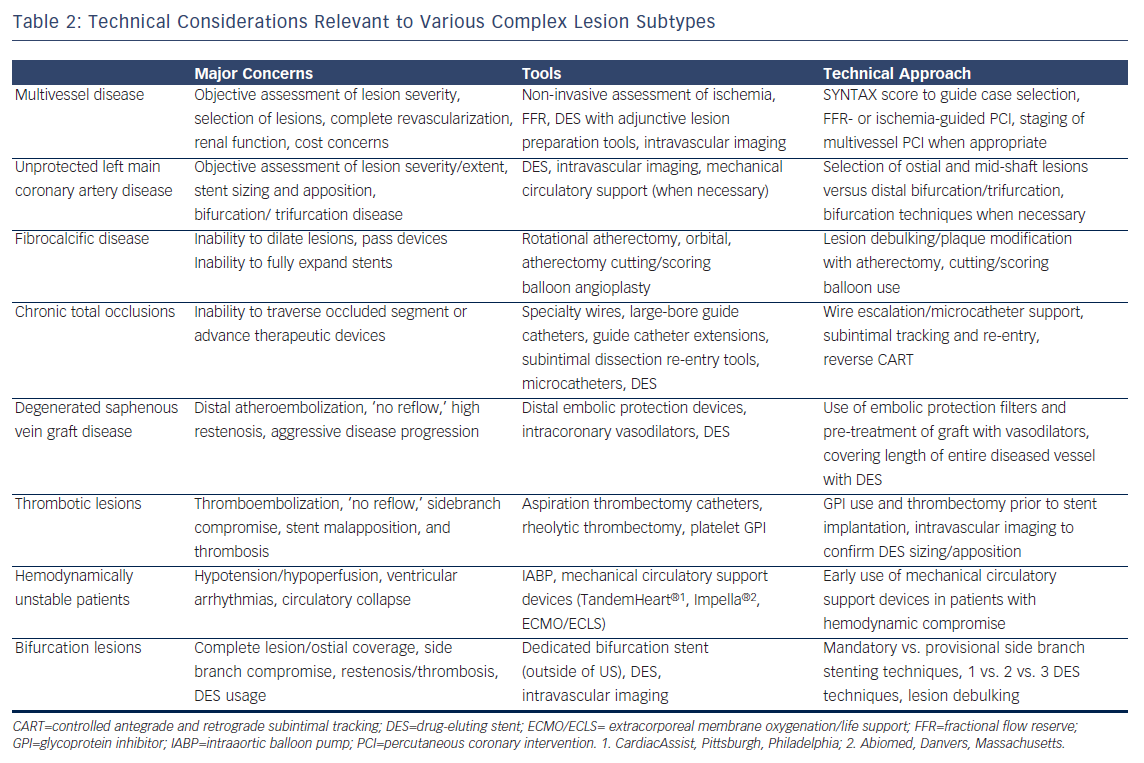
Challenges in contemporary catheter-based therapy for CAD generally stem from one or more of the following factors: the extent, severity, distribution, and characteristics of the coronary lesions, number of vessels diseased, LMCA involvement, presentation acuity and procedural urgency, burden of ischemia, hemodynamics/ventricular function, and medical comorbidities. Specific lesion sets that are associated with lower rates of procedural success and higher rates of recurrence or major adverse cardiac events (MACE) include multivessel disease, unprotected LMCA disease, fibrocalcific or undilatable lesions, chronic total occlusions, degenerated saphenous vein graft lesions, thrombotic lesions, hemodynamically unstable patients, and bifurcation/trifurcation disease. Broad technical considerations relevant to each of these lesion subtypes are summarized in Table 2, with bifurcation disease also addressed below in greater detail. In a published Dynamic Registry PCI experience that predated the advent of DES, the majority (55.1 %) of attempted lesions fulfilled at least one of the aforementioned criteria for complexity with over a quarter of lesions demonstrating two or more complex characteristics.7 Similarly, following the introduction of DES in the US in 2003, investigators from the EVENT (Evaluation of Drug Eluting Stents and Ischemic Events) Registry found that the majority (60.2 %) of intervened lesions fulfilled either ACC/AHA B2 or C lesion criteria.16 Thus, a large proportion of contemporary PCI procedures invoke some measure of technical complexity. While it is beyond the scope of this article to discuss each of the aforementioned complex lesion subtypes in detail, suffice it to say that tools and validated strategies currently exist for each scenario listed. It is incumbent upon the operator aspiring to tackle complex disease in the catheterization laboratory, to gain intimate familiarity with these data and technical strategies.
Bifurcation Disease—Classification and Percutaneous Therapeutic Options
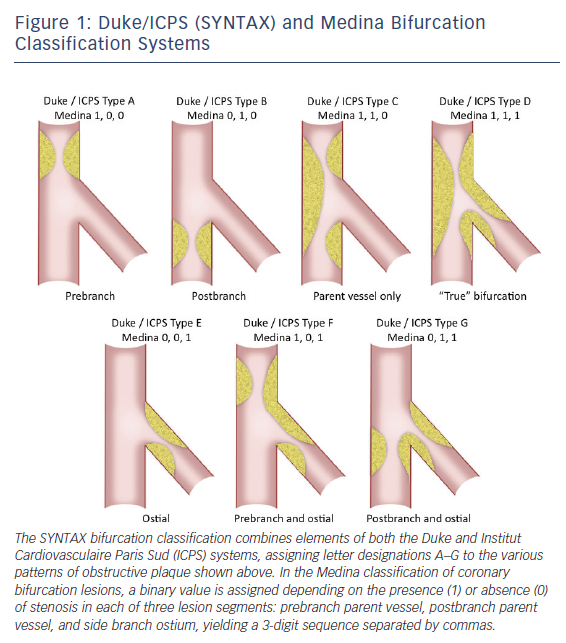
Within the spectrum of complex coronary lesions approachable by PCI, bifurcation disease merits special consideration as it is encountered frequently, accounting for 15-25 % of PCIs in some series, and has been associated with higher-than-average technical complexity and lower success rates.7,17,18 Optimal percutaneous treatment of bifurcation disease is guided by an extensive body of bench and clinical investigation with available data bearing out the potential consequences of inappropriate treatment, such as restenosis and/ or thrombosis of one or both vessels involved. Multiple bifurcation classification systems have been developed with the common goal of clarifying optimal interventional strategy and predicting complication risk.17–20 All schemas quantify the extent and location of plaque burden with some also incorporating the angle between parent and daughter vessel. The SYNTAX bifurcation classification, modified from the wellknown Duke and Institut Cardiovasculaire Paris Sud (ICPS) criteria, along with the Medina classification, representing a contemporary, simplified system, are shown in Figure 1.13,20,21 Side branch angulation is missing from both of these classification systems, although it is now well-recognized as an additional metric with important prognostic value.21 Whichever the system applied, ‘true’ bifurcation disease is characterized by obstructive disease in the parent vessel, pre- and post-side branch, as well as obstructive disease within the ostium of the side branch.
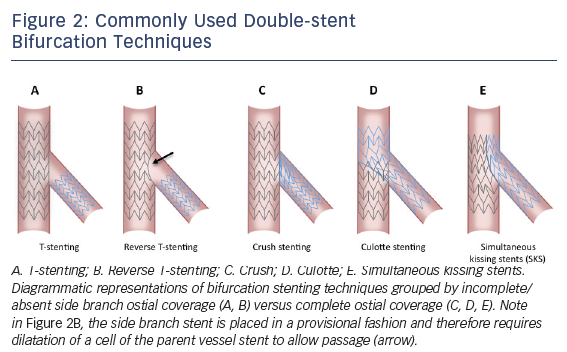
Even more numerous than bifurcation classification systems are the technical approaches described to date, varying widely in terms of the number of stents mandatorily used, completeness of coverage of the side branch ostium, and procedural complexity. A consensus classification of families of bifurcation techniques was proposed by the European Bifurcation Club (EBC) some years ago.21,22 This system, referred to as the MADS classification, is an acronym with each letter corresponding to a different choice for first vessel/segment addressed and approach to initial stent deployment. ‘M’ stands for Main proximal vessel first, ‘A’ for main Across side branch first, ‘D’ for Distal first, and ‘S’ for Side branch first. Various bifurcation techniques, including those double-stent techniques detailed in Figure 2 along with several others, are categorized under each lettered group and further broken out by the use of one, two, or three stents. Two-stent techniques that do not insure complete side branch coverage include the variations on the T-stent technique (see Figure 2) including classical and reverse T-stenting. More advanced techniques that allow for complete side branch coverage include variations on crush stenting, culotte stenting, and classical or modified simultaneous kissing stent (SKS) techniques.17,20,22
The results of numerous published clinical trials and registries of bifurcation technique have been evaluated in the context of several meta-analyses.23–31 These systematic reviews have found with great consistency that in the current era of DES, a simple, single-stent strategy using provisional side branch stenting, when feasible, is superior to complex (double stent) strategies with respect to rates of myocardial infarction and stent thrombosis.23–31 If a satisfactory angiographic result is obtained with parent vessel stenting ± side branch ballooning, forgoing side branch stenting is appropriate based on the available data and, moreover, will save on procedural time and cost, radiation exposure, and contrast usage.17,20–22 As fractional flow reserve (FFR) was demonstrated to be an important discriminatory tool for guiding the performance of single- or multivessel PCI in the Fractional Flow Reserve versus Angiography for Multivessel Evaluation 2 (FAME-2) study, so too has the value of FFR been demonstrated in assessing the functional significance of jailed side branch stenoses.32 Ahn et al. studied 230 jailed side branch stenoses in bifurcation lesions where main vessel stenting was performed and found that only 17.8 % of jailed side branch lesions were associated with functional significance (FFR <0.80).33 Moreover, visual discrimination of ‘significant’ side branch stenoses by angiography alone was limited at best.
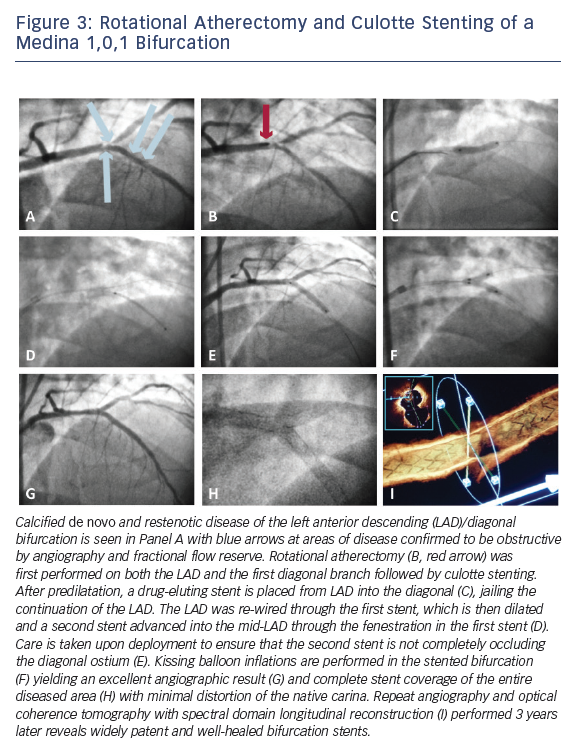
However, specific situations exist where one may wish to commit early to a complex bifurcation strategy. Intermediate to large side branches (>2.5 mm diameter), particularly those that are comparably sized as the parent vessel, side branches evidencing contiguous obstructive disease extending away from the ostium, side branch territories with demonstrable ischemia, or significant/flow-limiting dissection may merit consideration of a more complex bifurcation strategy with deliberate stenting of the side branch. Figure 3 depicts step-wise detail of a culotte stenting procedure in which calcified de novo and restenotic disease in the bifurcation of an LAD and large diagonal branch warranted a complex, multistent approach following debulking with rotational atherectomy. In planning percutaneous therapy for complex bifurcation disease, careful pre-procedure consideration of the coronary anatomy, aforementioned criteria, and various technical strategies, is therefore warranted.17,20–22
Cost-effectiveness Considerations in Routine and Complex Percutaneous Coronary Intervention
When broadly considering the cost impact of treatment strategies in patients with CAD, multiple therapeutic comparisons are of clinical and fiscal relevance. The first set of considerations relates to medical management versus revascularization in the setting of stable CAD. The next relates to mode of revascularization, surgical versus percutaneous, with the additional matter of routine versus selective use of DES in the latter group. In the interest of brevity, we will focus on cost-effectiveness of various revascularization strategies as it relates to patients with complex disease. While it is beyond the scope of this article to explore economic modeling in detail, it bears mention that variability and complexity of cost modeling methodology, differences in individual costs within the US healthcare system and across countries, and local trends in the practice of IC have all contributed to the lack of uniformity in conclusions regarding the cost-effectiveness of various revascularization strategies.34
Since commercial approval in the US in 2003, use of DES has grown, peaking in late 2005 at nearly 90 % and since settling into its current usage rate in over two-thirds of PCI procedures.35 Numerous randomized and non-randomized comparisons of BMS versus DES in PCI have been conducted and have uniformly found a reduction in target vessel revascularization (TVR) without significant reduction in death or myocardial infarction.36,37 Available economic analyses have not, however, uniformly upheld the cost-effectiveness of DES use in contemporary PCI. As noted, given the lack of mortality benefit with DES, the economic case to be made in favor of DES usage rests primarily with the ratio of incremental cost of these devices over BMS to enhanced quality of life (QoL) for patients who enjoy greater freedom from repeat revascularization following DES implant.38 Groeneveld et al. conducted a systematic review of the published literature on costs and QoL metrics associated with DES versus BMS use, incorporating eight QOL and four cost publications.38 In this analysis, patients receiving DES had $1,600 to $3,200 higher initial costs with the 1-year total cost differential dropping to $200 to $1,200. Wide variability in the relative rates of restenosis between BMS and DES in the studies included drove the large observed range in cost per revascularization avoided ($1,800–$36,900). Although all included studies were in agreement that restenosis negatively affects QOL, routine use of DES to avoid restenosis was found unlikely to be cost-effective.
In another systematic review of DES cost-effectiveness, Ligthart and colleagues similarly found wide variability in the reported cost-effectiveness of DES that the authors concluded was influenced by the quality of the studies analyzed, source of study funding, and the country in which the studies were conducted.34 Ryan et al. have suggested however that DES usage would be economically favorable if used selectively in patients at moderate to high risk of BMS restenosis with sensitivity analyses demonstrating an acceptable cost-effectiveness ratio of < $10,000 per repeat revascularization avoided if the expected BMS TVR rate in a given population exceeded 11 % and cost savings if the BMS TVR rate exceeded 19 %.39 As noted, use of FFR guidance in single or multivessel PCI with implantation of second-generation DES in the FAME-2 trial yielded substantial reductions in the ischemic composite endpoint over optimal medical therapy (4.3 % in the PCI group and 12.7 % in the medical therapy group, hazard ratio [HR] with PCI 0.32; 95 % confidence interval [CI] 0.19 to 0.53; p<0.001).32 An economic analysis of these data found that while initial costs of drug-eluting stent PCI performed in the setting of FFR <0.80 were significantly higher compared with FFR followed by optimal medical therapy ($9,927 versus $3,900; p<0.001), the observed $6,027 difference decreased over the study’s 1-year follow-up to $2,883 (p<0.001), offset by the cost of subsequent revascularization procedures in the medical therapy arm. The incremental cost-effectiveness ratio (ICER) of PCI guided by an abnormal FFR in FAME-2 was $36,000 per quality-adjusted life year (QALY), an economically favorable value as it is below the standard willingness to pay threshold of $50,000 per QALY.40 Taken together, these data indicate that cost-containment strategies in PCI should include objective assessment of functional significance to guide lesion selection and estimation of restenosis/revascularization risks to help guide the use of DES versus BMS along with strategies to minimize the number of stents implanted and experience-based choices regarding adjunctive device use.
Relevant to the economics of complex PCI, a few recent studies have re-examined the age-old controversy of CABG versus drug-eluting stent PCI in multivessel CAD. As mentioned above, the SYNTAX trial randomly assigned 1,800 patients with multivessel or unprotected LMCA disease to CABG surgery versus PCI with paclitaxel-eluting DES. Twelvemonth rates of major adverse cardiac or cerebrovascular events were significantly higher in the PCI group (17.8 % versus 12.4 % for CABG; p=0.002), primarily due to an increased rate of repeat revascularization (13.5 % versus 5.9 %; p<0.001) with no difference in all-cause mortality, thus failing to demonstrate non-inferiority between the two treatment arms.15 However, when outcomes were stratified by tertiles of SYNTAX score there was noted to be an interaction between the SYNTAX score and treatment allocation with comparable MACE rates between PCI and CABG in those subjects with low (0–22) or intermediate (23–32) scores. A formal cost-effectiveness analysis conducted by Cohen et al. based on the SYNTAX data found that in the overall study population total costs for the index procedure and hospitalization were $5,693/patient higher in the CABG group, but follow-up costs $2,282/patient higher in the PCI group (driven primarily by the need for repeat TVR), thus economically favoring PCI at 1 year despite high resource utilization for PCI (average 4.5 DES per procedure; range 0–14 DES).41 Although PCI was deemed to be the economically dominant strategy in the primary analysis, disease complexity as quantified by tertiles of SYNTAX score once again served as an interaction term. The 1-year cost savings with PCI diminished from $6,154/patient among patients with low SYNTAX scores to $3,889/patient in patients with intermediate SYNTAX scores to $466/patient in patients with high SYNTAX scores. A similar interaction was also found in terms of disease complexity and qualityadjusted life expectancy with CABG strongly favored in patients with the highest SYNTAX scores. In 1,900 patients with diabetes randomized to drug-eluting stent PCI versus CABG in the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM), total 5-year costs were similarly $3,641 higher per CABG patient. However, when the trial data were projected over a lifetime survival horizon, CABG posted significant gains in quality-adjusted life expectancy relative to PCI.42 Careful assessment of up-front costs, anticipated intermediate- and long-term outcomes, and the need for repeat procedures and hospitalization must therefore accompany technical planning of revascularization in patients with complex multivessel CAD.
Percutaneous chronic total occlusion (CTO) revascularization is another sector of contemporary interventional practice that has recently seen renewed interest and utilization driven by advances in technology as well as the development of hybrid percutaneous treatment algorithms.43 Limited data exist regarding cost-effectiveness of percutaneous revascularization of CTOs versus medical management and, at the time of writing, no formal cost-modeling versus CABG exists although the presence of one or more CTOs is often cited as the primary reason for CABG referral.44 Gada et al. used a decision-analytic model to evaluate the morbidity and costs associated with CTO PCI versus optimal medical therapy in patients with Canadian Cardiovascular Society class III–IV angina.45 Assuming a reference case mean age of 60 years and CTO PCI success rate of 67.9 % and 5 years of simulated follow-up, along with literature-defined assumptions regarding procedural probabilities, costs, and outcomes, CTO PCI was more costly than optimal medical therapy ($31,512 versus $27,805), but resulted in greater QALYs (2.38 versus 1.99), thus resulting in an economically favorable ICER of $9,505 per QALY. As experience grows with use of the hybrid CTO algorithm as well as with current strategies for tackling bifurcation lesions with conventional DES or with dedicated bifurcation stent systems available outside the US, additional cost modeling data addressing these complex PCI subsets will hopefully be forthcoming.46
Conclusions
Technically complex PCI procedures, while increasingly performed, remain associated with lower rates of procedural success and higher rates of MACE compared with more straightforward catheter-based interventions. Multivessel and unprotected LMCA disease, fibrocalcific lesions, chronic total occlusions, and bifurcation disease comprise many of the lesion sets requiring additional resource allocation, procedural planning, and sophistication. Bifurcation lesions, in particular, have been the subject of intense systematic study and some degree of controversy. Current consensus supports a simple, single-stent/provisional side branch strategy when possible. Cost considerations in PCI are perhaps most relevant to patients with extensive, multivessel disease in whom CABG may also be a viable therapeutic option. Objective assessment of disease complexity, estimation of technical feasibility, and consideration of medical comorbidities should all factor into the decision regarding optimal revascularization strategy.