Presentation 1: Impella 5.0/5.5 in the Setting of Post-cardiotomy Failure
Presented by: Hermann Reichenspurner, MD, PhD
Dr Reichenspurner started his presentation by listing the indications of Impella 5.0/5.5 as follows:
- Cardiogenic shock;
- Weaning from extracorporeal membrane oxygenation (ECMO);
- Bridge-to-bridge before left ventricular assist device (LVAD) implantation or before heart transplant;
- Bride to recovery: myocarditis, peripartum; and
- Cardiac surgery
- Mitral valve surgery;
- Aortic valve surgery; and
- Coronary artery bypass graft (CABG)/off-pump coronary artery bypass (OPCAB) with left ventricular (LV) ejection fraction (EF) <30%.
He emphasised the importance of avoiding low cardiac output syndrome (LCOS) following cardiac surgery (post-cardiotomy cardiogenic shock), which is caused by a transient decrease in systemic perfusion secondary to myocardial dysfunction. LCOS occurs in 10–15% of all cardiac surgery and is associated with poor clinical outcomes and mortality. Dr Reichenspurner referred to a study conducted on a population of 774,881 CABG patients from the Society of Thoracic Surgeon (STS) database that aimed to describe and test the STS risk model for CABG.1 That study found that patients with reduced EF undergoing cardiac surgery have an increased rate of LCOS.1
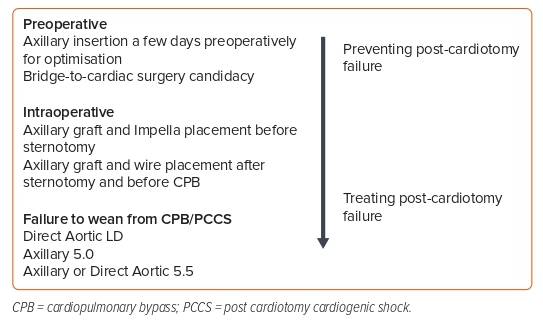
There are numerous different ways in which Impella support can be used in cardiac surgery that aim to prevent post-cardiotomy failure or, if it has occurred, treat it (Figure 1). Preventing post-cardiotomy failure can be achieved by preoperative axillary insertion of the Impella for optimisation or as a bridge to cardiac surgery candidacy. The Impella can also be used intraoperatively via axillary graft placement pre-sternotomy or with axillary graft and wire placement after sternotomy and before cardiopulmonary bypass (CPB). In patients with failure to wean from CPB, the Impella pump can be placed directly either through the ascending aorta or the axillary artery.
Dr Reichenspurner presented a patient case to illustrate the use of Impella in the treatment of post-cardiotomy failure: a 63-year-old male patient underwent aortic valve replacement (AVR) 10 years earlier. The patient deteriorated with an aortic valve opening area of 0.8 cm2, an EF of 62% and a left ventricular end-diastolic diameter (LVEDD) of 68 mm. Due to his coronary anatomy, the patient was not suitable for transcatheter aortic valve replacement (TAVR). He underwent a redo AVR and, due to the failure to wean off CPB, an Impella 5.0 was implanted. The patient was extubated after 4 h, fully mobilised on the device on Day 1 postoperatively and the Impella explanted under local anaesthesia on Day 8. At this time, the patient was doing well with an EF of 30% and an LVEDD of 60 mm.
In a second patient case, a 74-year-old male patient had been experiencing dyspnoea for 4 weeks. A coronary angiogram confirmed triple-vessel disease, and the patient’s EF was 22% and LVEDD 68 mm. His cardiac output deteriorated to 2 l/min, but percutaneous coronary intervention (PCI) was not considered appropriate due to severe peripheral arterial vascular disease. The patient underwent a protected OPCAB graft with the use of Impella 5.5 during the procedure. Dr Reichenspurner has performed nine OPCABs with Impella so far and has achieved 100% complete revascularisation and in-hospital survival. His patients were able to be mobilised on support from Day 1 postoperatively.
Dr Reichenspurner outlined the trials that are coming up: IMPACT (Impella Protected Cardiac Surgery Trial) US was presented at the A-CURE 2022 annual symposium and IMPACT EU is an upcoming trial to examine the use of Impella in high-risk cardiac surgery.2 The PRIME (Protected CABG with Impella) trial is a German multicentre trial on OPCAB with the use of Impella under preparation. Dr Reichenspurner finished by looking forward to the results of these studies in the near future.
Presentation 2: Impella 5.5/5.0 as a Bridge to Durable Left Ventricular Assist Device
Presented by: Alexander Bernhardt, MD
Dr Bernhardt reported the outcomes from the STS Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) 2020 Annual Report that reviewed 25,551 patients undergoing primary isolated continuous-flow LVAD implantation per annum between 2010 and 2019.2 In 2019, 50% of patients were INTERMACS Profile 1 or 2 before durable LVAD, and 73% received an LVAD as destination therapy.2 The 1- and 2-year survival rates in the most recent era have improved compared with the period from 2010 to 2014 (82.3% and 73.1% versus 80.5% and 69.1%, respectively; p<0.0001).3 Major bleeding and infection continue to be the leading adverse events. The incidence of stroke has declined in the current era to 12.7% at 1 year.3
Dr Bernhardt recapped the evidence that demonstrated Impella 5.0/5.5 is a feasible bridge-to-decision option for patients following extracorporeal life support (ECLS) implantation and allows further evaluation of a patient’s neurological state and future therapy needs.4 Dr Bernhardt proceeded with an overview of a multicentre retrospective study that gathered data on 531 patients who underwent durable mechanical circulatory support implantation after ECLS between January 2010 and August 2018 in 11 high-volume European centres.5 That study found that patients receiving durable mechanical circulatory support after ECLS experienced significant survival benefit with 1 year survival of 53%.5 Another study demonstrated the benefits of the Impella 5.0/5.5 device over ECLS as a bridging device before LVAD implantation, which included evaluation of right ventricle (RV) function and early mobilisation and optimisation of patients.6 Dr Bernhardt asserted that using Impella enables full haemodynamic assessment and facilitates better prognostic and therapeutic strategies.
This concept was subsequently proven in a feasibility study of nine patients who were implanted with an Impella 5.0 after ECLS.7 Survival of 90% was achieved. Following CE approval, the first 46 consecutive patients underwent Impella 5.5 implantation in six German centres between March 2018 and September 2019. Of those patients who underwent bridge-to-durable LVAD implantation, a 30-day survival rate of 89.5% was achieved.8
Building on this evidence, Dr Bernhardt set up a retrospective multicentre study in Germany to collect the outcomes and adverse events in patients who were bridged with axillary Impella pumps to durable LVAD. The goal of the registry is to assess the predictors of poor outcomes, especially right heart failure (RHF). Dr Bernhardt presented the preliminary results of this study, which have not yet been published. One-third of the study subjects were resuscitated, with 40% implanted with ECLS before Impella support. In this very sick patient population, the 1-year survival rate was >70%.
Panel Discussion
Dr Anderson thanked the experts for their presentations and kicked off the discussion with the first question. In respect of high-risk cardiac surgery, patients are often steered towards high-risk PCI and surgery is left for the truly high-risk patients who have no alternative options. Dr Anderson asked the experts how they decide which patients are appropriate for high-risk PCI and which are appropriate for high-risk cardiac surgery.
Dr Reichenspurner responded that, in his clinical experience, the majority of high-risk patients are generally better off with PCI, but there is a proportion of high-risk patients for whom PCI is not suitable or they have contraindications. He believed that a defined strategy is required for this population of very high-risk patients. Dr Reichenspurner is interested in the results of the IMPACT pilot trial because it examines this high-risk patient group and compares prophylactic Impella with standard care to determine whether Impella will improve current outcomes of high-risk cardiac surgery.
Dr Anderson anticipated that Impella will make a difference and agreed that the results from the IMPACT pilot trial will be exciting. He anticipated not only positive survival rates, but also a positive impact on acute renal injury, ventilator time and duration in the intensive care unit (ICU).
Dr Anderson continued by asking the experts how they determine whether to insert the Impella upfront or intraoperatively, or whether they wean the patient before implantation.
Dr Reichenspurner expressed that we have learnt that the aortic balloon pump only works if used prophylactically, whether that is primarily or while on bypass, but he would not use it belatedly. Dr Anderson was in agreement with this approach. In response to Dr Reichenspurner’s enquiry about the use of Impella in off-pump coronary artery surgery, Dr Anderson replied by describing the advantages of avoiding bypass and reducing global ischaemic time. He confirmed that off-pump surgery can impair the RV during manipulation of the LV, but with Impella the LV is decompressed, which reduces compromise of the RV during cardiac manipulation. He added that it is, of course, always possible to put the patient on a pump acutely if necessary.
Dr Bernhardt was in agreement with the views of Drs Reichenspurner and Anderson. He added that patients who decompensate in the ICU have the worst outcomes, so it is important to take steps to prevent post-cardiotomy cardiac failure for these patients in particular. One step he takes to minimise this risk is to use the Impella device intraoperatively. In this way, the Impella can help stabilise the patient not only intraoperatively, but also in the crucial 48 h after surgery. Further, he finds that the Impella improves renal function and can achieve early mobilisation of the patient, enabling surgeons to decide when to safely wean the patient off support.
Dr Anderson asked the panellists about the pros and cons of direct aortic versus axillary insertion.
Dr Reichenspurner replied that the great advantage of axillary insertion over direct insertion is that its removal can be performed in the ICU rather than in the operating room. He offered a patient example in which the left axillary approach was taken because right side access was impossible. In his clinical practice, the aortic approach will always be the last choice.
Dr Anderson added that there are alternatives to direct insertion when removing the graft because it is not always necessary to perform a redo sternotomy to explant it, and it is therefore helpful to have both options available.
Dr Anderson next enquired about the mechanism of pre-LVAD implantation and how it improves patient outcomes.
Dr Bernhardt explained that the Impella 5.5 can be used as a bridging device to provide time to improve the overall condition of the patient and optimise other systemic issues, such as cardiac state, fluid status and end organ function. This means that the patient can be brought out of the acute phase of cardiogenic shock and the risk of infection can be mitigated so that the patient is left only with LV-sided failure prior to durable LVAD implantation.
Dr Reichenspurner added that, at present, durable LVADs are implanted into INTERMACS Profile 1 and 2 patients, and half of these patients survive but half do not. In order to make the LVAD more acceptable as a treatment, he believes it is important to stabilise and improve the patient condition before implantation, in the same way patients are stabilised prior to undergoing heart transplant. The Impella 5.5 achieves this by buying the patient time during which they can recover and mobilise. The better the condition the patient is in, the better the outcomes are likely to be after LVAD implantation.
Dr Anderson enquired about the techniques used by the panellists to minimise axillary artery injury and the risk of stroke during the explant of the Impella device during durable LVAD implantation.
Dr Bernhardt replied that in his practice the extraction technique has recently been updated and he now recommends extraction of the implant from the apex instead of the axillary artery. He advised closing by manipulation of the carotids to minimise the risk of stroke. In his practice, he has not experienced any incidents of stroke using this updated practice method.
Dr Reichenspurner agreed with this recommendation. He considered removing the Impella via the apex a useful change of technique to use whenever the opportunity presents, and especially if the support duration was for a prolonged period of time.