Transcatheter aortic valve implantation (TAVI) has been demonstrated in randomised trials to be associated with a lower risk of death and disabling stroke compared with surgery.1 Therefore, it is likely that TAVI will increasingly become the preferred treatment modality for most patients with symptomatic severe aortic stenosis, regardless of baseline surgical risk. However, as TAVI begins to enter younger, lower-risk cohorts, the prevalence of bicuspid aortic valve (BAV) will increase, and it is important that clinicians recognise that this patient subset was excluded from all clinical trials comparing TAVI and surgery.2 Although clinical outcomes of TAVI in BAV have improved with newer generation devices, the incidence of paravalvular regurgitation and of conduction disturbance remains higher than with surgery, and both of these complications have been associated with adverse long-term outcomes.3–6
Therefore, in the absence of randomised data directly comparing these two treatment modalities, careful patient selection by the Heart Team, based on clinical and, perhaps more importantly, anatomical characteristics, must remain paramount. Furthermore, optimising transcatheter heart valve (THV) sizing and positioning within bicuspid anatomy remains an important consideration.
With this as a background, one potential solution to the challenges of TAVI in BAV is patient-specific computer simulation. In this review, we discuss how patient-specific computer simulation may be used to guide the transcatheter treatment of patients with BAV.
Patient-specific Computer Simulation
Patient-specific computer simulation is a method of simulating the interaction between a device and the native anatomy.7 The simulations use the geometric and mechanical properties of both the device and patient anatomy to predict the deformation of the device and the potential for important procedural complications. The technology has been studied in a number of structural heart procedures, including TAVI, left atrial appendage occlusion and transcatheter mitral valve replacement.8–20
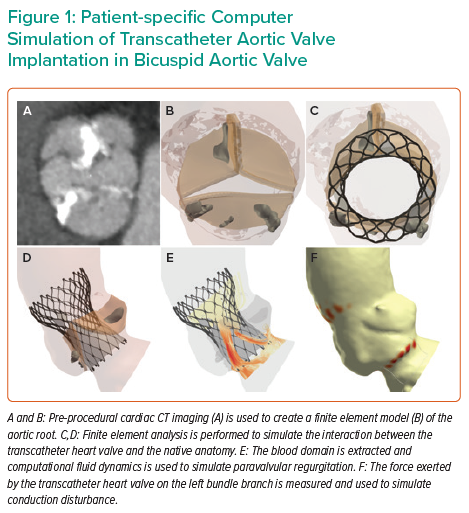
Patient-specific computer simulation of TAVI in BAV has been undertaken in a number of small studies, but the largest validation and prospective clinical experience has been performed using the FEops HEARTguide technology.11,12,21–25 An overview of patient-specific computer simulation of TAVI in BAV is presented in Figure 1.
Finite Element Analysis
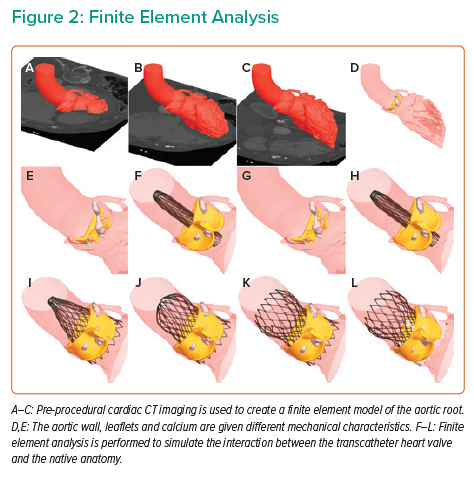
The first step in patient-specific computer simulation is to create a finite element model of the aortic root (Figure 2 and Supplementary Material Video 1). Pre-procedural cardiac CT imaging is segmented with inclusion of the left ventricular outflow tract, aortic root and the ascending aorta. The aortic root tissues, leaflets and calcification are modelled with different elastic material properties.13,26 These material characteristics were derived through a process of iterative back-calculation from 39 tricuspid aortic valve patients who had pre- and post-procedural cardiac CT.15 Aortic leaflet modelling has been developed for both Sievers type 0 and type 1 BAV.27 Furthermore, modelling can be performed to reflect both tricommissural, bicommissural raphe-type and bicommissural non-raphe-type leaflet morphology.28
Finite element models have been developed for a number of THVs, including SAPIEN XT (Edwards Lifesciences), CoreValve, Evolut R, Evolut PRO, Evolut PRO+ (Medtronic), Lotus and ACURATE neo (Boston Scientific). The frame morphology is derived from micro-CT, or based on information shared by the device manufacturer. The strut width is obtained from optical microscopy, or again, based on information shared by the manufacturer. Mechanical properties of the nickel titanium (Nitinol) frames are obtained through in vitro radial compression testing, with radial force recorded throughout the compression and unloading cycle.
Pre-dilatation is simulated and then the finite element model of the THV is positioned within the aortic root model. Finite element analysis (FEA) is performed to simulate the interaction between the THV and the native anatomy.
Computational Fluid Dynamics
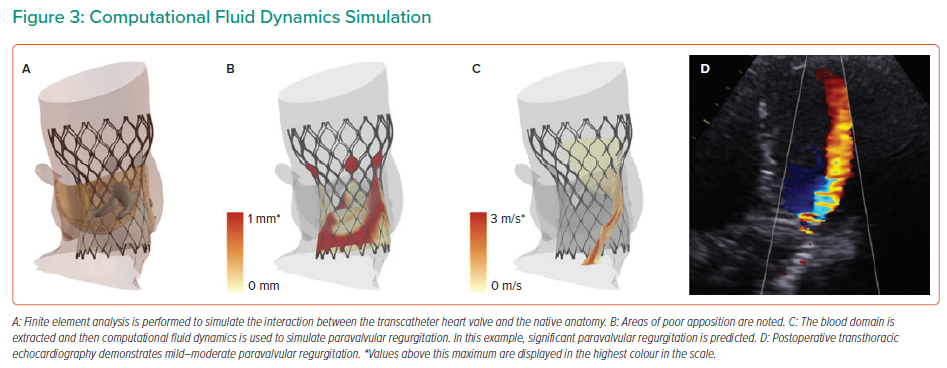
An overview of the computational fluid dynamics (CFD) is presented in Figure 3. The blood domain is first extracted from the FEA output. A fixed pressure gradient of 32 mmHg is applied across the aortic root, a value that was derived using invasive measurement from 60 patients with tricuspid aortic valve.14 CFD is performed and paravalvular regurgitation is recorded in the left ventricular outflow tract. Importantly, the CFD simulations do not model for valvular regurgitation. The simulations are performed with the bioprosthetic leaflets aligned with the native leaflets, given that non-alignment of the bioprosthetic leaflets has been demonstrated to have minimal impact on the computer simulation output.16
Conduction Disturbance Modelling
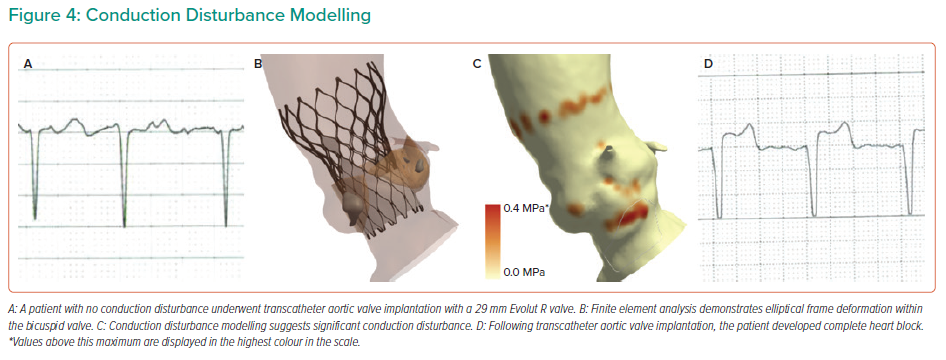
Conduction disturbance modelling is presented in Figure 4. The inferior border of the membranous septum is located and then a region extending to 15 mm below the aortic annulus is identified. This anatomical region of interest is a surrogate for the His bundle and proximal left bundle branch.29 The force exerted by the THV on the native anatomy is extracted from the FEA modelling. The percentage of the region of interest that is subject to pressure (contact pressure index) is recorded. Importantly, the simulations do not account for differences in THV frame rotation, given that this has been demonstrated to have minimal impact on the computer simulation output.13
Validation of Computer Simulations
Retrospective validation of the computer simulations has been performed in 37 patients who had pre- and post-procedural cardiac CT imaging, with further validation performed on seven patients who had peri-procedural transoesophageal imaging.9,12 The majority of these patients had Sievers type 1 BAV (82%). The FEA simulations were found to be reliable at predicting the THV frame deformation within the bicuspid anatomy.
Validation of the CFD analysis has demonstrated that the simulations are reliable at predicting the development of moderate paravalvular regurgitation. A predicted paravalvular regurgitation of 13.6 ml/s was found to be 92% sensitive and 72% specific for predicting the development of moderate paravalvular regurgitation, representing a positive predictive value of 61% and a negative predictive value of 95%.
Limited validation of the conduction disturbance modelling has been performed in 20 patients. A contact pressure index of 0.14 was found to have a sensitivity of 67% and a specificity of 72% for predicting the development of major conduction abnormalities (new left bundle branch block, Mobitz type II second-degree atrioventricular block or third-degree atrioventricular block), representing a positive predictive value of 100% and a negative predictive value of 50%.
Optimising Transcatheter Heart Valve Sizing and Positioning
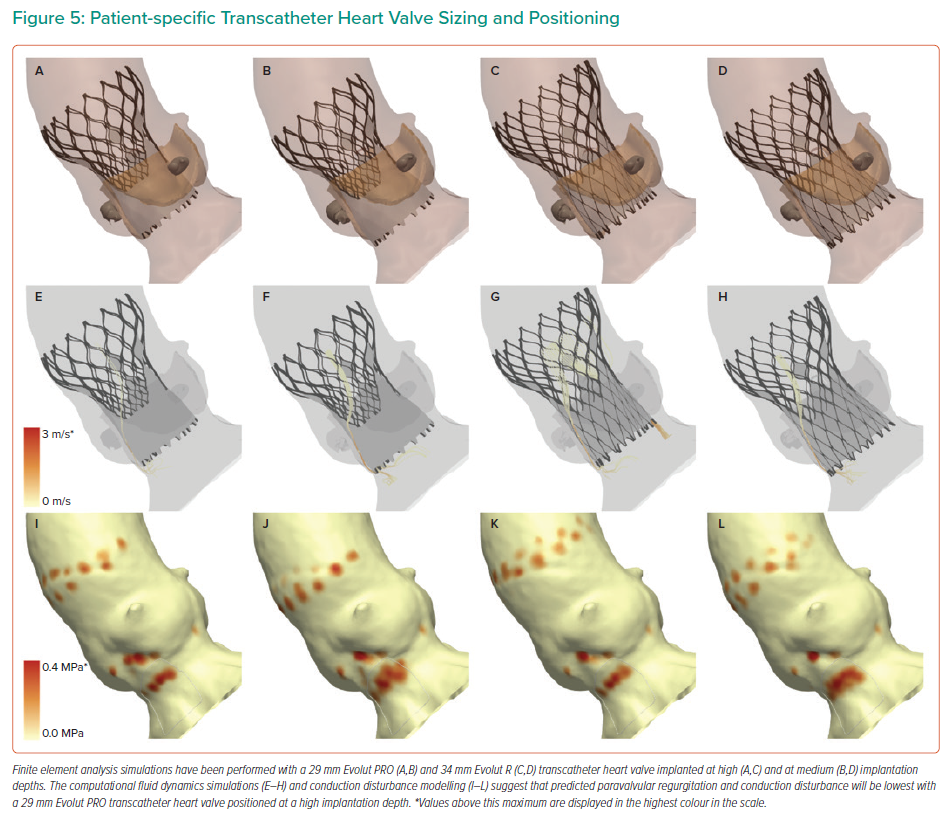
The computer simulations may be used to optimise THV sizing and positioning to reduce the severity of predicted paravalvular regurgitation and conduction disturbance (Figure 5).30 Simulations are performed using a THV sized using the perimeter-derived aortic annulus dimensions, and additional simulations may also be undertaken using a ‘downsized’ THV. Furthermore, simulations may also be performed with the THV positioned at multiple different implantation depths, typically at a high (0 mm), medium (4 mm), and, in some select cases, a deep (8 mm) implantation depth. Operators may then review the multiple simulations to choose the optimal THV size and implantation depth that reduces predicted paravalvular regurgitation and conduction disturbance.
Prospective Experience
To date, the clinical outcomes of 19 patients who have been treated prospectively using the technology have been presented.11,25 A significant number of these patients had tricommissural leaflet morphology (47%), but patients with bicommissural raphe-type (37%) and bicommissural non-raphe-type (16%) leaflet morphology were also studied (Table 1). The computer simulations have been demonstrated to be a useful aid for guiding treatment decisions in BAV patients. In particular, the CFD analysis may be used to identify patients whose aortic root anatomy may not be suitable for TAVI because of the potential to develop significant paravalvular regurgitation. In prospective clinical usage, the computer simulations have suggested that five patients might develop significant paravalvular regurgitation if treated with a self-expanding THV, and, following discussion in the heart team meeting, four of these patients were treated with surgery. One patient was thought by the heart team to have a too high risk for surgery and was treated with a balloon-expandable THV.
For the 14 patients who were treated with TAVI using a self-expanding THV, the computer simulations were found to be a useful guide for THV sizing and positioning, and in 10 of these patients, procedural aspects were altered to minimise paravalvular regurgitation. In one of these patients, a deep implant was predicted to minimise paravalvular regurgitation and therefore a pre-procedural permanent pacemaker was implanted, given that the computer simulations predicted significant conduction disturbance with a deep implantation depth.
Favourable clinical outcomes have been observed in all 19 patients, with no patient developing moderate paravalvular regurgitation and no patient requiring post-procedural implantation of a permanent pacemaker.
Limitations
It is important that clinicians recognise the limitations of computer modelling. The FEA is not performed in a pressurised state and so is currently unable to simulate important procedural complications such as THV embolisation. Furthermore, the aortic root tissues are modelled with elastic material properties and therefore the FEA is unable to simulate aortic root rupture. The correct modelling of aortic leaflet morphology is dependent on high-quality cardiac CT imaging studies.
Only limited validation of the FEA has been performed for patients with Sievers type 0 (bicommissural non-raphe-type) BAV. The CFD simulations do not have perfect diagnostic accuracy. Conduction disturbance modelling may not be feasible in all patients, given that adequate right-sided contrast opacification is required. Although multiple computer simulations may be performed to model a variety of different THV positions, achieving a target implantation depth may not always be feasible with current-generation self-expanding devices, because of the risk of device migration or embolisation on release.31 Furthermore, repositioning of the THV may induce trauma to the conduction tissue, although this risk might be mitigated by the top-down deployment technique, which is being evaluated in the Optimize PRO study (NCT04091048). The FEops HEARTguide technology is currently unable to model the SAPIEN 3 THV (Edwards Lifesciences), which has been associated with favourable procedural outcomes in bicuspid anatomy, although patient-specific computer simulation of BAV with this THV has been performed by other groups.22,32 Due to time and financial constraints, use of the technology may not be feasible in all patients, and its usage could potentially be limited to patients with high-risk complex anatomical subsets, such as patients with a calcified raphe or excess leaflet calcification, or for patients with a tapered aortic root anatomy, for whom THV downsizing might be considered.33,34
Future Directions
It is important to consider how patient-specific computer simulation of BAV could best be implemented into routine clinical practice. Ideally, technologies such as deep learning would be used to rapidly identify patients with BAV at the time of cardiac CT acquisition.35 Cardiac CT imaging might then be transferred onto a cloud-based platform, where deep learning algorithms could assist with automatic segmentation of aortic root structures, reducing case processing times.36,37 Computer simulation output could then be provided to treating physicians in an expedited manner.38
To date, limited prospective experience exists with patient-specific computer simulation of TAVI in BAV. To address this shortcoming, the PRECISE TAVI study (NCT04473443) will examine the role of patient-specific computer simulation in heart team decision-making in complex anatomy.
Optimal THV device selection for bicuspid anatomy is yet to be established and patient-specific computer simulation could potentially be used to guide the selection of a high-radial-force or high-conformity THV.21
Ultimately, there exists the potential for patient-specific THVs to be developed, which would be tailored to the patient’s precise anatomical characteristics and could potentially reduce both paravalvular regurgitation and conduction disturbance.26
Finally, further studies will be needed to determine if hospital outcomes of TAVI in BAV patients might benefit from other uses of artificial intelligence, such as deep learning, which has been demonstrated to be predictive of short-term outcomes in other fields of cardiovascular diseases, such as carotid artery stenting.39
Conclusion
As the use of TAVI continues to expand into the younger, lower-risk patient cohort, achieving excellent clinical outcomes in BAV is important. Patient-specific computer simulation is an emerging technology that may be used by the heart team to guide the transcatheter treatment of patients with BAV.
Click here to view Supplementary Material Video.
Clinical Perspective
- Patient-specific computer simulation of transcatheter aortic valve implantation in bicuspid aortic valve may predict the development of important clinical outcomes such as paravalvular regurgitation and conduction disturbance
- Patient-specific computer simulation may be used to optimise transcatheter heart valve sizing and positioning to minimise predicted paravalvular regurgitation and conduction disturbance
- The usage of patient-specific computer simulation to risk-stratify bicuspid patients and to optimise transcatheter heart valve sizing and positioning has been associated with favourable clinical outcomes