A 33-year-old man who had undergone an uneventful arterial switch operation (ASO) to treat dextro-transposition of the great arteries at the age of 1 week presented with crescendo angina. He had no conventional risk factors for coronary artery disease (CAD) apart from tobacco smoking. He had no other significant past medical history and took no regular medications.
On presentation, his blood pressure was 130/80 mmHg, pulse rate was 75 BPM, temperature was 37.0oC, respiratory rate was 18/min and oxygen saturation was 100% on room air. A 12-lead ECG showed sinus rhythm with no ST-T changes. The laboratory work-up revealed haemoglobin of 15.8 g/dl (11.5–15.5 g/dl), white cell count 6.7 × 103/mm3 (4−11 × 103/mm3), platelet count 173 × 103/mm3 (150−440 × 103/mm3), urea 37 mg/dl (10–50 mg/dl), creatinine 1.0 mg/dl (0.3–1.4 mg/dl), high-sensitivity cardiac troponin 0.009 ng/ml (<0.014 ng/ml), serum triglycerides 230 mg/dl (40–160 mg/dl), total cholesterol 156 mg/dl (<200 mg/dl), LDL cholesterol 75 mg/dl (<130 mg/dl) and HDL cholesterol 35 mg/dl (35–55 mg/dl). Values in parentheses represent reference ranges or upper reference limits.
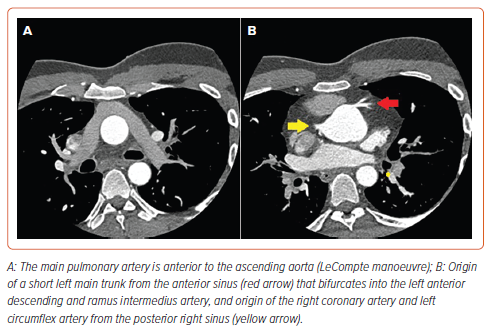
Multi-slice CT coronary angiography (MSCT-CA) showed the right coronary artery (RCA) and the left circumflex artery (LCx) arising from the posterior right sinus with separate origins, resembling the non-coronary sinus in normal anatomy. The LCx had a retro-aortic course (Supplementary Video 1). The left main coronary artery was seen arising from the anterior-facing sinus, resembling the right coronary sinus in normal anatomy, then bifurcated into left anterior descending (LAD) and ramus intermedius arteries (Figure 1). The LAD showed proximal severe stenosis.
Cardiac MRI showed average indexed volumes of both ventricles with preserved systolic function as well as mild mitral and tricuspid regurgitation (Supplementary Video 2), with no late gadolinium enhancement.
After heart team discussion, a decision was made to proceed with coronary angiography and percutaneous coronary intervention (PCI). One of the anticipated technical challenges was engagement of the coronary arteries due to the abnormal orientation and takeoff.
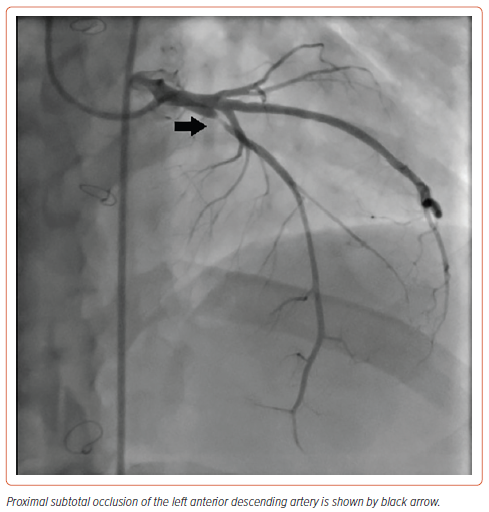
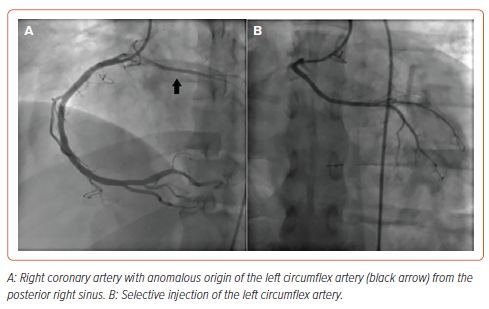
After reviewing the MSCT-CA images, the decision was made to use the femoral rather than the radial approach to facilitate catheter manipulation and engagement of the coronaries. We chose to start with an Amplatz Left 1 (AL1; Merit Medical) diagnostic catheter. The RCA was engaged in the left anterior oblique view with counterclockwise rotation. The LCx was engaged more posterior to the RCA origin. The left main coronary artery was engaged by applying excessive clockwise rotation to the AL1 catheter. The LAD showed proximal subtotal occlusion (Figure 2). The ramus intermedius artery, LCx and RCA showed no significant lesions (Figure 3).
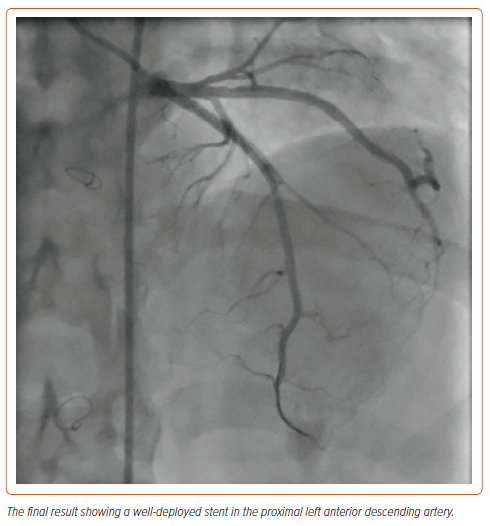
PCI was done using the AL1/6 Fr guiding catheter. Direct stenting of the LAD was performed using a 3.5 × 28 mm everolimus-eluting stent followed by post-dilatation using a 3.5 × 15 mm non-compliant balloon with excellent final results (Figure 4). The patient was discharged 1 day later on dual antiplatelet therapy, a high-intensity statin and bisoprolol. On follow up after 3 months, he was asymptomatic.
Discussion
The ASO has been the standard surgical intervention for dextro-transposition of the great arteries since the late 1980s, with long-term survival rates of up to 95% after 25 years.1 This has resulted in a growing population of adult ASO survivors.
The most challenging step in the ASO is coronary reimplantation, making coronary-related events a common cause of early mortality and morbidity after the procedure. Late complications still occur, with a cumulative probability of 12% over a 15-year follow-up period.2 Data about long-term outcomes beyond 25 years are limited.
Different mechanisms have been proposed for the development of premature CAD after an ASO.3 Progressive dilatation of the aortic root may cause kinking, stretching or stenosis of the reimplanted coronary arteries.4 The reconstruction of the pulmonary artery during the LeCompte manoeuvre may cause extrinsic compression of either coronary artery. Whether these manoeuvres increase the risk of premature atherosclerosis is still uncertain, but accelerated intimal thickening has been reported in otherwise normal coronary arteries after ASO.2
Intravascular ultrasound studies in this patient population have demonstrated proximal intimal thickening in 89% of patients.5 Anomalous origin of the LCx from the RCA, although reported to be of little clinical significance, may also be associated with increased risk of atherosclerosis.6
In our case, the coronary ostia were free from kinking or compression, suggesting accelerated atherosclerosis rather than a direct sequela of coronary reimplantation. Although there was a traditional risk factor for atherosclerosis (tobacco smoking), the young age and absence of family history of premature CAD may point to an accelerated mechanism for atherosclerosis.
ASO survivors with CAD may be asymptomatic due to surgical coronary denervation, with the first presentation being sudden cardiac arrest.7 It is reasonable to perform MSCT-CA or invasive coronary angiography in asymptomatic adults to evaluate coronary patency and identify those at high risk.8,9 Because MSCT-CA is classically acquired during a diastolic phase, coronary compression and angulation during systole may be missed or underestimated. Therefore, careful analysis during the entire cardiac cycle is recommended.
In most reported cases of CAD after an ASO, surgical revascularisation was the procedure of choice.10 Techniques included coronary artery bypass grafting or pericardial patch coronary arterioplasty. A recent systematic review of 993 adult ASO survivors found only four reported cases of patients who underwent coronary intervention, of whom three had surgical revascularisation, while only one patient, who was aged 18 years, had a drug-eluting stent inserted in the RCA due to external compression by patch pulmonary arterioplasty.11,12
To the best of our knowledge, this is the first reported case of coronary atherosclerosis that was managed by PCI more than 30 years after an ASO.
Coronary catheter engagement is challenging after an ASO due to the unusual orientation of coronary sinuses. There is no specific technique described to engage the coronary arteries. In this article, we provide technical details on catheter selection and manipulation after an ASO.
We suggest performing MSCT-CA routinely before cardiac catheterisation for proper visualisation of the coronary sinuses and a full evaluation of the anatomy of the aortic root and coronary arteries. Discussion between multidisciplinary team members, including cardiac imagers, grown-up congenital heart disease specialists, interventional cardiologists and surgeons is essential for individualised decision-making while considering the patient’s preferences.
Conclusion
While CAD in ASO survivors is usually related to coronary reimplantation or external compression by the pulmonary artery, accelerated atherosclerosis may play an important role. More studies are required to determine the exact prevalence and underlying mechanism(s). Routine screening for coronary artery disease in this patient population could be useful in early detection and prevention of cardiovascular events. Pre-procedural MSCT-CA is helpful to guide systematic planning for PCI.