The invasive measurement of fractional flow reserve (FFR) can determine the haemodynamic relevance of coronary artery stenoses. Determination of FFR is recommended in coronary artery stenoses with a luminal diameter narrowing between 50 % and 90 % if no non-invasive proof of ischaemia is available.1 To measure the FFR of a given coronary lesion, a wire or a microcatheter equipped with a miniaturised pressure sensor is inserted into the coronary artery. Under conditions of maximum hyperaemia, the relationship between the mean blood pressure distal to the stenosis (pd) and mean pressure in the aorta (pa) is determined. Generally, FFR values ≤0.80 are ance and associated prognostic relevance of the respective stenosis.
Based on the results of several randomised, prospective clinical studies, in which the decision to perform revascularisation was based on FFR, the method carries high clinical relevance. In the absence of non-invasive proof of ischaemia, FFR performed in stenoses with a 50–90 % diameter reduction was given a “class I recommendation” and “level of evidence A” in the guidelines for coronary revascularisation published by the European Society of Cardiology in 2014.2 The latest update of the American College of Cardiology/American Heart Association Guideline for the Diagnosis and Management of Patients with Stable Ischemic Heart Disease states: “It has been suggested in several studies that a PCI (percutaneous coronary intervention) strategy guided by FFR may be superior to a strategy guided by angiography alone”.3 One large clinical registry demonstrated that performing FFR changed further management in 43 % of patients compared to the purely visual assessment of coronary artery stenoses by invasive angiography.4 Even when non-invasive proof of ischaemia is available, FFR measurements often change clinical judgement regarding the need to revascularise a given coronary artery stenosis.5 However, despite clear evidence and guidance, many interventional cardiologists continue to rely on the visual assessment of stenosis severity alone, rather than performing FFR.6 Potential reasons include the logistical effort of performing FFR, concerns regarding potential complications and uncertainty about optimal performance and interpretation of FFR measurements, particularly in complex situations, such as multivessel disease, left main stenoses, serial stenoses or in patients with aortocoronary bypass grafts. In addition, while performing FFR is not technically difficult per se, several relevant procedural aspects have to be taken into account in order to avoid incorrect measurements or misinterpretation of results.
For this reason, the present study provides a summary of consensus recommendations regarding the indication, performance and interpretation of FFR measurements. Clear evidence from randomised studies is not available for all patient groups and clinical situations. Therefore, this expert consensus is based on the most pertinent literature and the authors’ personal experience with the procedure. Particular emphasis is placed on the practical performance of FFR measurements, along with tips and tricks for everyday clinical practice.
Clinical Evidence
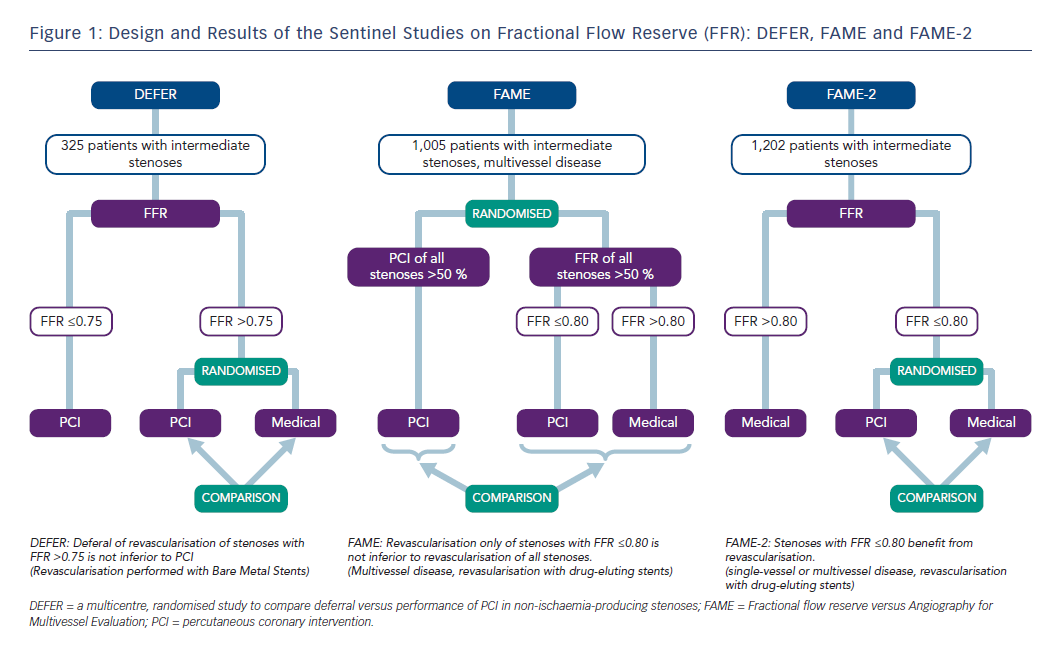
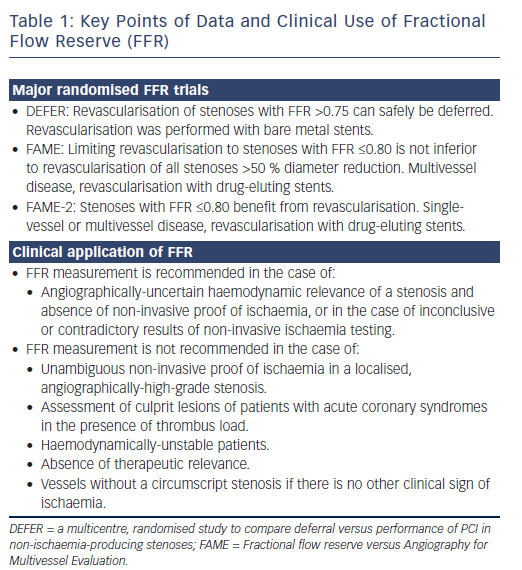
Revascularisation guided by FFR in patients with coronary artery disease and stenoses >50 % leads to better outcomes than revascularisation based on a visual analysis of angiographic stenosis severity alone. This is the conclusion of three large, randomised trials: the DEFER (a multicentre, randomised study to compare deferral versus performance of PCI in non-ischaemia-producing stenoses) trial, the Fractional flow reserve versus Angiography for Multivessel Evaluation (FAME) trial and the FAME-2 trial (Figure 1), including long-term follow up of patients initially included in these studies.
The DEFER trial, published in 2001, included patients with de novo stenoses of intermediate angiographic severity.7 If FFR was ≤0.75, percutaneous coronary intervention (PCI) was performed. Patients with FFR >0.75 were randomised to PCI (perform PCI group, n=91) or conservative therapy (defer group, n=90). Regarding the primary endpoint survival free of major adverse cardiac events (MACE), there was no difference between the two groups after 1 and 5 years (perform PCI versus defer group: 73 % versus 80 % after 5 years, p=0.052).8 The composite rate of cardiac death and acute myocardial infarction in the perform PCI group was 7.9 % compared to 2.2 % in the defer group (p=0.021). This leads to the conclusion that a stenosis of intermediate angiographic severity, and with an FFR value >0.75, can be treated with a conservative approach. The risk of myocardial infarction or cardiac death was less than 1 % per year in patients treated conservatively. Even follow up after 15 years (complete in 92 % of patients) demonstrated that there was no increased event rate in patients with deferred PCI. In fact, the defer group had a significant advantage in the incidence of myocardial infarction (2 % versus 10 %). Regarding mortality and the number of revascularisations, no significant differences were observed between the two groups.9
The FAME trial, published in 2009, randomised 1,005 patients with twoor three-vessel coronary artery disease to angiographically- (n=496) or FFR-guided revascularisation (n=509).10 In the angiographically-guided arm, all stenoses ≥50 % were revascularised, while in the FFR-guided arm, PCI was only performed when FFR was ≤0.80. The mean number of stents placed was 2.7±1.2 per patient in the angiographically-guided group, and the primary endpoint (a combination of death, myocardial infarction and revascularisation) occurred in 18.3 % of patients after 1 year. In patients randomised to FFR-guided treatment, a mean of 1.9±1.3 stents per patient were implanted, and the primary endpoint occurred in only 13.2 % of individuals (p=0.002). Mortality was not significantly different between the two groups. The rate of a combined endpoint composed of death and myocardial infarction, albeit not prespecified, was significantly lower in the FFR-guided group after 2 years of follow up (8.4 % versus 12.9 %, p=0.002).11 Five-year data confirmed the long-term safety of the FFR-guided PCI strategy in patients with multivessel disease.12
While the DEFER and FAME trials clarified that revascularisation could safely be deferred if FFR indicates the absence of haemodynamic relevance, the FAME-2 trial, published in 2012, investigated whether patients profit from revascularisation if FFR is pathological (here: ≤0.80).13 The FAME-2 trial included stable patients with one-, two- or three-vessel disease. All lesions >50 % were investigated by FFR. Patients with stenoses with an FFR value ≤0.80 were randomised to PCI versus purely conservative therapy. Patients with FFR values >0.80 in all vessels were included in a registry. After randomisation of 880 patients and the inclusion of 322 patients into the registry, the FAME-2 trial was terminated prematurely, as the primary endpoint (combination of death, myocardial infarction and urgent revascularisation) among patients with an FFR ≤0.80 occurred significantly less frequently in the PCI group compared to the group managed with medication (4.3 % versus 12.7 %, hazard ratio with PCI: 0.32, p<0.001). The difference was mainly driven by the endpoint “urgent revascularisation” (1.6 % versus 11.1 %, hazard ratio with PCI: 0.13, p<0.001).13 Two-year follow-up data showed that the advantage of FFR-guided PCI compared to drug therapy was sustained.14
Therefore, the DEFER and FAME trials demonstrated that, in patients with stable coronary artery disease, the conservative management of stenoses that could be angiographically severe, but are not haemodynamically relevant, is safe. The DEFER trial used a threshold of 0.75 to define haemodynamic relevance by FFR, but it mainly included patients with single-vessel disease, and bare metal stents were used for revascularisation (so that positive effects of revascularisation were less likely than with modern devices or in more severe disease). Patients randomised in the FAME trial had multivessel disease, PCI was performed with drug-eluting stents and the threshold value used for FFR was 0.80. The FAME-2 trial, in turn, demonstrated that patients with pathological FFR values (≤0.80) benefit from revascularisation with drug-eluting stents.
Large meta-analyses have evaluated the safety of using an FFRguided strategy. One meta-analysis encompassed a total of 49,517 patients, and showed a significantly lower rate of revascularisation (14.8 % versus 20.4 %), as well as a reduction of MACE (22.5 % versus 34.8 %), myocardial infarction (4.2 % versus 8.1 %) and death (7.6 % versus 15.3 %) in FFR-guided versus purely angiographicallyguided revascularisation.15 A second meta-analysis showed that FFRguided decision-making reduced the number of revascularisations by 50 % and the incidence of MACE by 20 % over a period of 16 months.16
When interpreting FFR, the range between 0.75 and 0.80 is often called the “grey zone”. Data from a large, ongoing observational study with 1,459 patients showed that classifying lesions with FFR values between 0.75 and 0.80 as “haemodynamically relevant” is justified, because even in this range, the long-term event rate after revascularisation using modern methods was lower than with purely drug-based therapy.17
Clinical Application of Fractional Flow Reserve
Even though the risk associated with measuring FFR is low, FFR measurement of a given stenosis should only be considered if revascularisation of that lesion with PCI or bypass surgery is possible in case of a positive result. Current guidelines recommend the use of FFR to determine the haemodynamic relevance of stenoses with an angiographic diameter reduction between 50 % and 90 %, for which no non-invasive proof of ischaemia exists.2 There could be indeterminate or contradictory findings of non-invasive ischaemia testing that can be clarified or resolved by the invasive measurement of FFR. If FFR demonstrates the absence of haemodynamic relevance, the risk of future events associated with a stenosis is low, and cannot be further reduced by PCI with modern stents. FFR measurements are of particular relevance in patients with multivessel disease. The anatomical and functional severity of individual lesions in such patients varies widely. The DEFER and FAME trials, as well as numerous other prospective investigations, showed that the FFR-guided revascularisation only of haemodynamically-relevant lesions is superior to the angiographicallyguided revascularisation of all lesions that appear as “severe” or “relevant” luminal stenoses (Table 1).
Performing Fractional Flow Reserve Measurements
Consent and Patient Preparation
Recommendations
To ensure that FFR can be performed within the context of any diagnostic angiogram if the need arises, it is recommendable to routinely include informed consent for FFR measurements into the consent procedure for every diagnostic angiogram or coronary intervention. Patients should be informed about potential discomfort (shortness of breath, angina, palpitations, sensation of heat, diaphoresis), as well as potential risks of the procedure (coronary injury caused by the guiding catheter or intracoronary wire, as well as side-effects of medication used to induce hyperaemia).
If intravenous application of medication to induce hyperaemia is planned, the patient should have a large venous access (either a sheath in the femoral vein or a venous cannula not further distally than the cubital vein). A more peripheral location could be acceptable if regadenoson is used. Sufficient flow via the venous access (e.g. by using parallel saline infusion) should be ensured in order to achieve rapid onset of medication. Because foreign material is being introduced into the coronary artery, we recommend anticoagulation and antithrombotic medication in the same way as for PCI when FFR measurements are performed.
Pitfalls, Tips and Tricks
Because patients typically do not interrupt their regular medication for invasive coronary angiography, and in many centres fasting is no longer required for invasive coronary procedures, the influence of drugs and nutrition on FFR measurements is a relevant issue. According to Ozdemir et al., beta blockers do not influence FFR measurement results, and therefore, do not need to be interrupted.18 The situation regarding caffeine is less clear. While caffeine and adenosine have antagonistic effects on A2a receptors, which could influence FFR results with adenosine-mediated hyperaemia, the majority of clinical studies, even including intravenous caffeine (4 mg/kg, corresponding to 3–4 cups of coffee), failed to demonstrate any significant effect.19 Therefore, the overall influence of caffeine appears to be minimal, especially when consumed in small amounts and >1 hour prior to FFR measurements.20,21 In case of doubt, alternative drugs to adenosine (e.g. papaverine) could be used. Theophylline should be interrupted at least 12 hours prior to FFR measurements.
Catheter Selection and Positioning
Recommendations
Transfemoral and transradial catheterisation are equally suited for FFR. For patient safety, the use of diagnostic catheters is not recommended. They should be exchanged for guide catheters (at least 5F) for FFR measurements, as coronary dissection by FFR wire is a theoretical possibility that would require immediate intervention. Catheters should not have side holes, as they could impair intracoronary adenosine administration. In addition, they could influence pressure calibration and equalisation due to local turbulences at the catheter tip. The catheter shape should be carefully chosen for coaxial alignment at the coronary ostium.
Pitfalls, Tips and Tricks
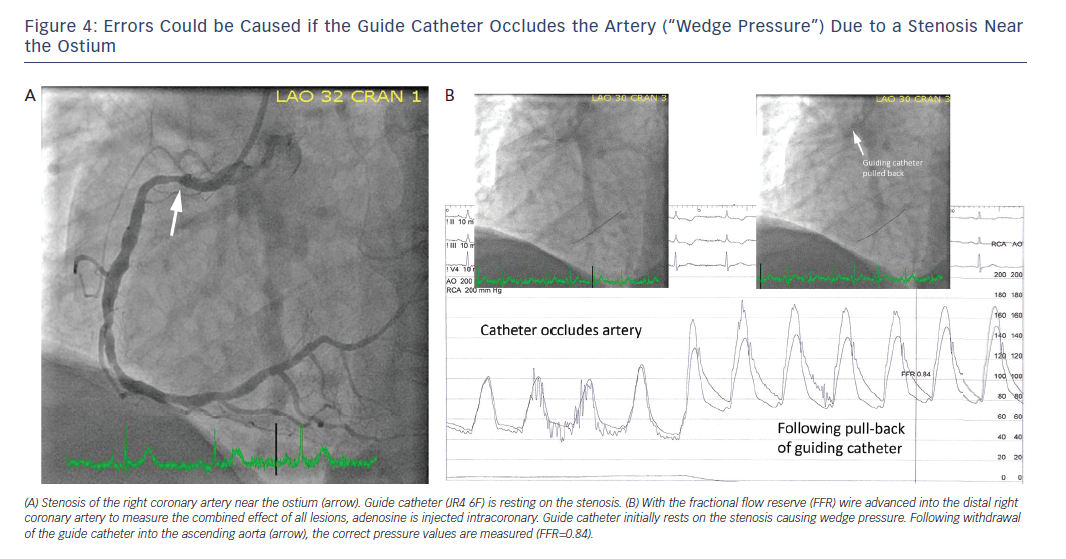
The catheter must not obstruct a narrow ostium or proximal stenosis (“wedge pressure”), as incorrectly low values could be measured for both pa and pd, which in turn would generate inaccurate FFR results. If in doubt, the catheter should be completely disengaged from the ostium for pressure calibration, equalisation and recording of pd/pa.
Calibration
Recommendations
As a first step, zeroing of the aortic pressure must be ascertained. For that purpose, the pressure transducer must be positioned at the level of the heart (1/3 versus 2/3 chest diameter), opened towards the atmosphere and zeroing of the pressure performed.
Next, the FFR wire/microcatheter is to be prepared and connected to the respective measurement system by cable or wireless connection. The following should be noted with regard to the different models:
- St Jude Aeris® wire: Lay the wire flat and flush the coil with saline solution. Then, keep wire stationary, press “connect wirelessly” on the recevier and activate the wire’s transmitter. The wire pressure sensor is zeroed automatically.
- Volcano Verrata® wire: Flush the wire, then connect. Zeroing of the pressure sensor then takes place automatically. When doing this, the catheter must be lying flat and not be moved.
- Acist Navvus® microcatheter: Flush the catheter, then connect.
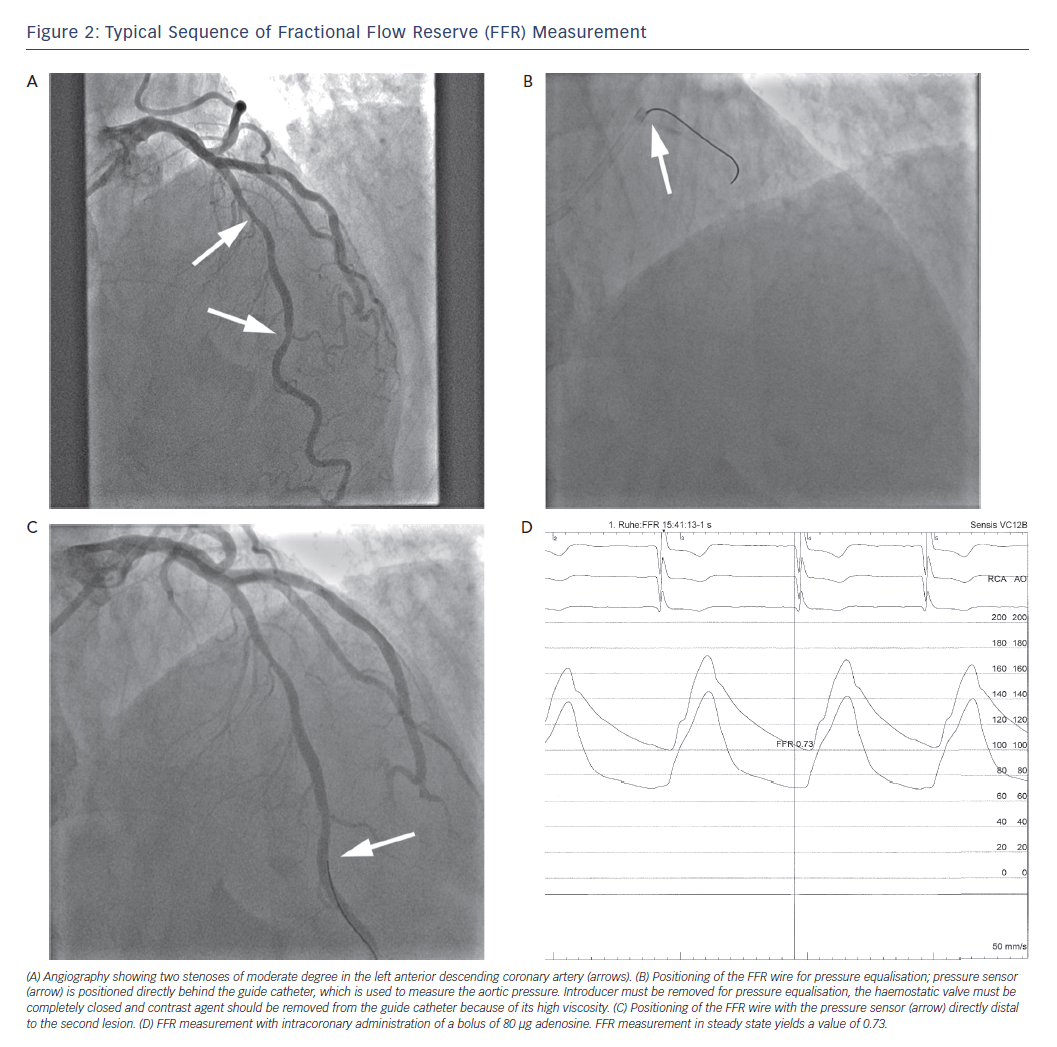
The wire (St Jude/Volcano) or the microcatheter (Acist) is then advanced into the coronary artery until the pressure sensor is positioned precisely at the end of the guide catheter (Figure 2). The guide catheter should be flushed at this point (due to the viscosity of contrast agent), and if necessary, disengaged from the coronay artery ostium. Pressure equalisation between the wire and aortic pressure must then be performed. This constitutes a procedural step of major importance.
Pitfalls, Tips and Tricks
For the equalisation of pressures between the pressure sensor and the aortic pressure, the introducer must be removed and the haemostatic valve completely closed. A second wire, in addition to the FFR wire, could result in artefacts due to mechanical interaction, and should therefore be avoided if possible. The pressure curves must be stable and free of artefacts for several heartbeats before zeroing is performed, because the mean pressure is generally averaged over three-to-five heartbeats.
“Drift” describes a slow deviation from baseline in the values measured by the pressure sensor (pressure wires/microcatheters generally have a specification of a maximum baseline drift of 7 mmHg/h); “shift” describes a sudden deviation from the baseline value, and can generally be traced back to connection problems or wire defects.
Positioning of the Fractional Flow Reserve Wire
Recommendations
The pressure sensor of the wire/microcatheter must be positioned directly distal to the lesion that is to be investigated. It should be placed in the main vessel, and not in a side branch. In case of sequential stenoses within one artery, the pessure sensor should be placed downstream of the most distal lesion (Figure 2).
Pitfalls, Tips and Tricks
The tip of the wire should be carefully shaped to an appropriate curve before introducing it (generally by no more than 45°). If disconnecting the wire to advance it into the coronary artery cannot be avoided, upon reconnection, artefact-free connection must be ensured.
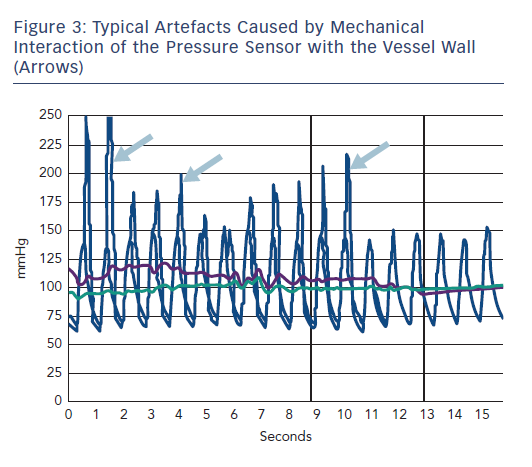
During measurement, it is important to be mindful of artefacts; the pressure sensor on the FFR wire could interact with the vessel wall, especially in cases of a small vessel caliber (Figure 3). Similarly, severely tortuous coronary segments can cause artefacts because of mechanical interaction of the pressure sensor with the vessel wall. Therefore, placement of the sensor in segments with substantial tortuosity should be avoided. It should also be noted that the presence of viscous contrast agent in the coronary artery can affect the gradient pd/pa. In the case of a resting gradient ≤0.80, haemodynamic relevance is evident, and hyperaemia is no longer required to make a clinical decision. If the FFR wire remains in the coronary vessel during an intervention, it should be ensured that the FFR wire is not “jailed” by stent implantation.
Hyperaemia
Recommendations
Prior to advancing the pressure wire/FFR microcatheter, nitroglycerin should be adminstered (intracoronary, generally 0.2 μg), in order to prevent spasms and minimise resistance in the epicardial vessels.
There are several pharmacological options and routes of administration to induce hyperaemia, as outlined below.
Intravenous Medication
Adenosine
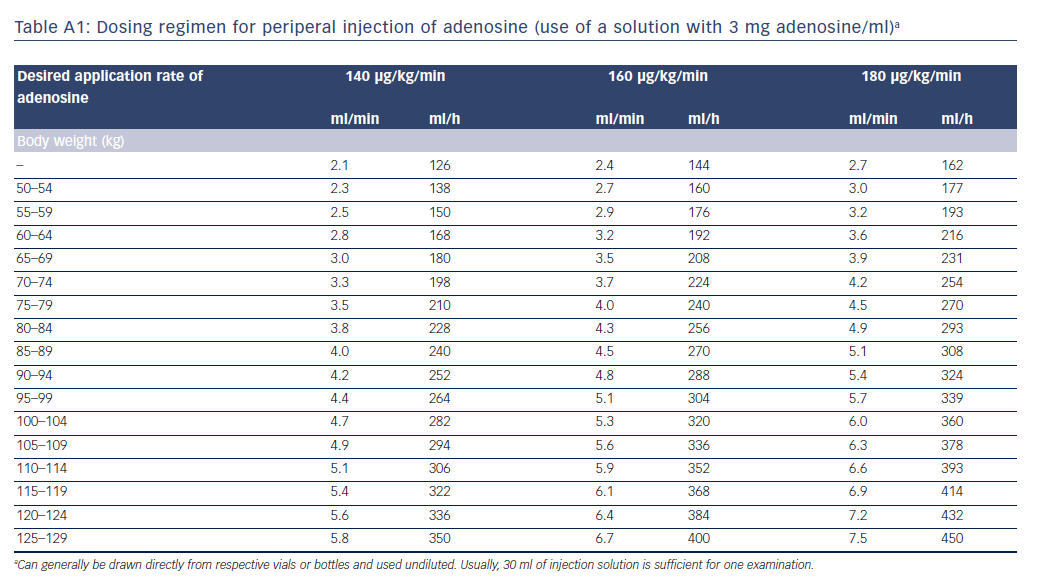
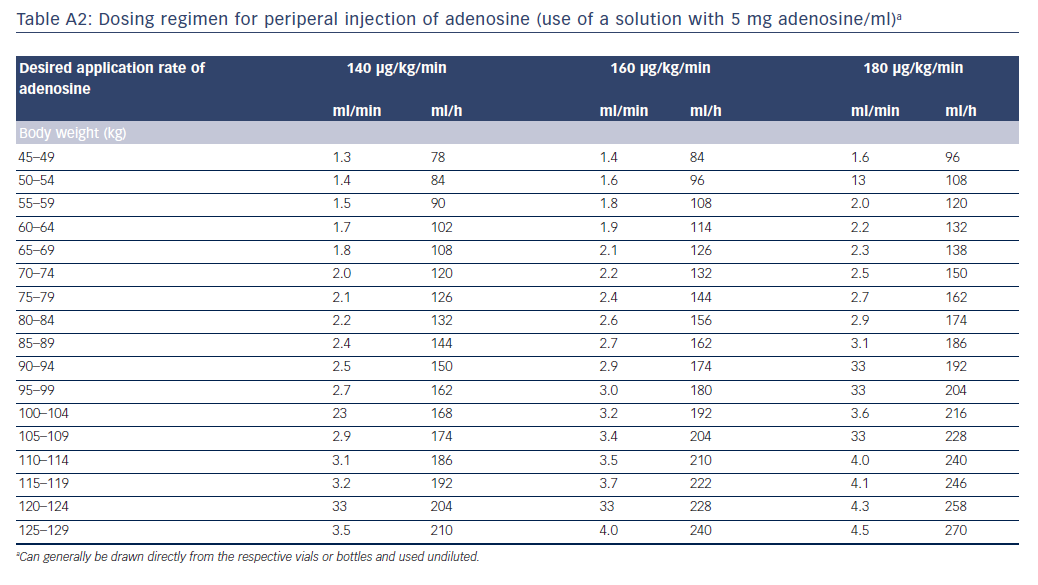
The intravenous administration of adenosine is safe and simple, provided that contraindications are observed. Given the very short half-life of adenosine, care must be taken to ensure that the site of administration is as proximal as possible, and flow speed is sufficient, otherwise adenosine could be degraded before it reaches the coronary circulation. The standard dose for sustained maximum hyperaemia is 140 μg/kg/min. Some authors recommend that if adenosine is not administered via a central venous access, the dose should be increased (e.g. 160–180 μg/kg/min), particularly if the FFR value is in the grey zone.22 The administration of more than 180 μg/kg/min is not recommended, because this could reduce coronary perfusion. Adenosine is commercially available at various concentrations (e.g. 3 mg/ml, 5 mg/ml). Tables A1 and A2 in the Appendix list exemplary rates of infusion depending on adenosine concentration, body weight and desired delivery rate.
Regadenoson
Regadenoson is administered intravenously at a standard dose of 400 μg, without adjustment to body weight. The time until onset of effect is approximately 37 seconds (compared to 66 seconds for intravenous adenosine.23). The maximal hyperaemic effect lasts for 30 seconds; hyperaemia fades out therafter, with a total duration of approximately 10 minutes. Overall, regadenoson appears to have fewer side-effects than adenosine and is considered to be safer in patients with chronic obstructive pulmonary disease.24 However, it has not been evaluated as thoroughly as adenosine.
Intracoronary Medication
Adenosine
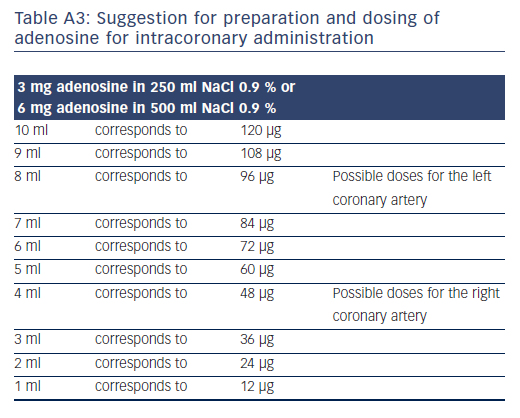
Adenosine doses for intracoronary administration vary from 40 μg to 200 μg, injected as a rapid bolus of, for example, 10 ml volume.25-27 As a general rule, lower doses should be used for the right than for the left coronary artery, as atrioventricular-block occurs more often. Doses of 80 μg for the left coronary artery and 40 μg for the right coronary artery yielded FFR results that correlated extremely closely to intravenous adenosine at 140 μg/kg/min (r=0.99).28 Table A3 in the online Appendix shows the options for creating a solution for injection dosing of intracoronary adenosine.
Papaverine
Papaverine (intracoronary 8 mg for the right coronary artery and 12 mg for the left coronary artery) is a possible alternative to adenosine.29 The onset of effect occurs after approximately 16 seconds. The duration of maximum hyperaemia is approximately 50±10 seconds, and return to the resting value occurs after approximately 2 minutes. Due to the lack of studies and potentially higher rate of side-effects (risk of accumulation and triggering of ventricular arrhythmias), papaverine should not be used as the standard substance for inducing hyperaemia.30
Pitfalls, Tips and Tricks
For the peripheral administration of adenosine, ready-to-use 50 ml vials for infusion in an undiluted fashion are available. Of note, injection pumps for the peripheral administration of adenosine must permit an injection rate >200 ml/min. Often, factory settings for the maximum possible injection rate are limited to lower values, and might need to be reprogrammed by the manufacturer. Administration of a contrast agent to induce hyperaemia is currently not recommended. If coronary spasm due to the FFR wire is suspected, repeated injection of nitrates might be required.
Registration and Evaluation
Recommendations
In the case of intravenous administration of the hyperaemic agent, pd/pa should be recorded until a steady state is reached, but for no less than 2 minutes. The FFR value is then the lowest ratio registered during the steady state (with the exception of artefacts that are not to be taken into account). It should be noted that the mean pd and pa often show a minimum just before reaching the constant mean pressure, but this point in time does not constitute the FFR value. In the case of intracoronary administration, pd/pa should be continuously recorded until it returns to the baseline value. The FFR value is the lowest recorded ratio (apart from artefacts).
When the FFR wire/microcatheter is withdrawn after completion of the measurement, maintenance of accurate calibration should always be verified when the pressure sensor reaches the guiding catheter. This is important to identify shift or drift of pressure readings, which would lead to erroneous FFR results. If this verification indicates a relevant deviation from the baseline value of 1.00, the measurement might need to be repeated.
Pitfalls, Tips and Tricks
In order to obtain correct aortic pressure tracings (pa), care must be taken to remove the wire introducer during the registration and to completely close the haemostatic valve. During FFR registration, the guide catheter must not occlude the ostium or a stenosis close to the ostium. This would cause incorrectly low pressure values to be measured, which in turn results in false high FFR values (Figure 4). Outlier pd/pa values that are caused by artefacts or arrhythmia must be carefully observed, and not be taken into account. If the patient has ectopic beats, this can cause incorrectly low pd/pa values, which must be disregarded. Values measured under AV-block/bradycardia must also be classified as “not evaluable”. If AV-block occurs during intracoronary administration (especially in the right coronary artery), the measurement might need to be repeated with intravenous administration.
Documentation
Ideally, FFR measurements should be archived as pressure curves or as individually-measured numeric datasets.
Interpretation and Clinical Consequences
The FFR value indicates the extent of ischaemia. Generally, FFR values ≤0.80 indicate that the stenosis in question is haemodynamically relevant. Therefore, based on the data from the above-mentioned prospective clinical studies, revascularisation is recommended in the case of FFR values ≤0.80. If FFR values are >0.80, it is assumed that the lesion is not haemodynamically relevant, and as a general rule, there is no need for revascularisation.2
However, the results of FFR measurements must always be evaluated taking the clinical context and clinical information into account. If the results of the FFR measurement are in conflict with non-invasive proof of ischaemia or the patient’s symptoms, decisions regarding further management must be made on an individual basis. Ultimately, an FFR value of 0.80 does not represent a single, dichotomous threshold value to be used as the sole criterion to decide in favour of or against revascularisation (Tables 2 and 3).
Fractional Flow Reserve in Special Situations
Aorto-ostial Stenoses
In the presence of an aorto-ostial stenosis, the guide catheter must be removed from the ostium for equalisation of pressures and during hyperaemia. Intravenous administration of adenosine is preferable to intracoronary administration. Of note, the large, prospective, randomised studies on which the FFR recommendations are based excluded left main coronary artery stenoses and aorto-ostial stenoses. However, small, non-randomised studies suggest that the same threshold value (FFR ≤0.80) should be used for decision-making.31,32
Left Main Coronary Artery Stenosis
The best way to evaluate left main coronary artery stenosis is to take FFR measurements in both downstream vessels, unless one of them is very small. If one of the downstream vessels has a relevant stenosis, measurements should only be taken in the other vessel. If both downstream vessels have relevant stenoses, FFR evaluation of the left main coronary artery is not possible.33,34 Positioning of the pressure sensor before the downstream stenoses will not allow the left main coronary artery to be correctly evaluated.35;
Serial Stenoses
Serial stenoses cannot be evaluated individually in any straightforward fashion. In the case of two successive stenoses, the distally-measured value represents the combined effect of both lesions. As a pragmatic approach, it is recommended to first revascularise the lesion causing the higher pressure step-up upon pullback or in sequential measurements. If the higher pressure step-up cannot be clearly identified, the distal lesion should generally be treated first. Subsequently, the remaining stenosis must be reassessed.36–38
Long Vessel Segments without Circumscipt Luminal Stenosis in Angiography
FFR is only validated for stenoses with a degree of stenosis of at least 50 %. Lesion length is a significant predictor of a positive FFR.39,40 A pathological FFR value identifies ischaemia as a possible reason for symptoms, including vessels with long stenoses that do not appear to be relevant in the angiogram. Nevertheless, the therapeutic decision can be difficult. Careful pullback of the FFR wire/FFR microcatheter should be performed under continuous hyperaemia (or stepwise repeated measurements with intracoronary adenosine) in order to identify any localised pressure step-up. If such a pressure step-up is present, revascularisation should be considered, potentially in combination with intracoronary imaging. If no localised pressure stepup can be identified, revascularisation is generally not recommended.
Coronary Artery Bypass Grafts
There are limited data on FFR measurements in stenotic bypass vessels. In a non-randomised study, FFR-guided PCI of intermediate bypass graft stenoses (visually assessed to be 40–70 %) resulted in a lower MACE rate compared to an angiographic-guided strategy.41 Stenoses of native vessels downstream of a bypass graft could be considered as native stenoses, and should be assessable by FFR.
Acute Coronary Syndrome
In the case of ST-elevation myocardial infarction (STEMI), impaired microcirculation in the infarction region has been shown to influence FFR measurements in the culprit vessel for up to 6 months after the acute event.42 Despite data suggesting that FFR in the culprit vessel and its change over the first postinfarction days can predict functional recovery of the infarcted region,43 FFR measurements in culprit vessels are not currently recommended in STEMI patients. However, FFR has been used to guide revascularisation decisions in non-culprit vessels with a better outcome than angiographically-guided revascularisation.44
In patients with non-STEMI (NSTEMI), some trials have demonstrated that acute determination of the FFR in non-culprit lesions is safe, accurate and reproducible,45,46 and that it correlates with non-invasive proof of ischaemia or repeated FFR measurements after the acute phase.48,49 In addition, prospective studies (or subanalyses of such studies) have demonstrated the clinical value of FFR-based revascularisation in patients with NSTEMI.49–51 However, one observational analysis compared 206 consecutive acute coronary syndrome patients (NSTEMI and unstable angina) with 262 intermediate lesions to 370 stable coronary artery disease patients with 528 lesions. In that study, revascularisation was deferred if the FFR was >0.75. Not surprisingly, MACE rates were significantly higher in acute coronary syndrome patients than in stable patients, even when the FFR was >0.75. The best cut-off to predict future MACE was 0.81 in stable patients, but 0.84 in acute coronary syndrome patients.52 Similarly, a meta-analysis published by Adjej et al. identified an FFR threshold of 0.83 as the optimal cut-off to predict MACE in acute coronary syndrome patients (compared to 0.81 in patients with stable coronary artery disease).17
Overall, it can be assumed that, in the absence of clear impairment of microcirculation and relevant thrombus load, FFR measurements performed during immediate angiography are useful to plan further revascularisation in patients with acute coronary syndrome and multivessel coronary artery disease. Decisions about deferral of revascularisation might need to be made somewhat more cautiously for these patients compared to stable patients.
Myocardial Bridges
The administration of adenosine is not suitable for the evaluation of the possible haemodynamic relevance of myocardial bridges.53 Theoretically, dobutamine stress echocardiography would be more appropriate for this purpose.54 However, FFR measurements have not been evaluated for therapeutic decision-making in the case of myocardial bridges, the relevance of which is controversial.55
Fractional Flow Reserve Following Percutaneous Coronary Intervention
Assessing Revascularisation Success
In registries, FFR values after PCI correlate with the outcome.17,56 The higher the FFR value, the lower the event rate during follow up. A recent study of 574 patients with 664 lesions and FFR performed post-PCI suggested a threshold of 0.86 as the optimal predictor of MACE during follow up after PCI.57 In patients with acute coronary syndrome, that threshold might be higher (0.91 according to one study58). However, repeated FFR measurements are currently not routinely required or recommended post-PCI if the angiogram shows interventional success. If measurements are repeated, care must be taken with regard to shift and drift, and correct zeroing must be ensured. In particular, if the FFR wire was used for PCI and the pressure sensor remained distal to the lesion during the procedure, post-PCI FFR measurements must be followed by a careful pullback of the pressure sensor to the guide catheter to verify that correct calibration has been maintained.
Side Branch in the Case of Bifurcation Percutaneous Coronary Intervention
It is possible to assess the haemodynamic relevance of a side-branch stenosis caused by PCI using FFR. An FFR ≤0.80 in the side branch correlates with an increased rate of events during follow up. In the case of FFR >0.80, no side-branch intervention is required (Table 3).59-61
Instantaneous Wave-free Ratio and Pressure Distal to the Stenosis/Mean Pressure in the Aorta at Rest and without Hyperaemia
The need to use vasodilator substances to induce hyperaemia could be perceived as a limiting factor for FFR measurements and could prevent adoption in clinical routine. Two alternative pressuremeasurement methods, which are not based on hyperaemia, have been described in the literature. First, evaluation of the pd/pa ratio without hyperaemia has been suggested. Second, the so-called “instantaneous wave-free ratio” (iFR) has been proposed. It represents the pd/pa ratio not during the entire cardiac cycle, but during a specific phase within diastole when resistance in the microvasculature is lowest. All commonly-used FFR systems enable resting pd/pa values to be recorded across the entire cardiac cycle. Determination of the iFR requires a special algorithm, and is currently only possible with specific software (Volcano Harvest®).
The diagnostic accuracy of iFR compared to standard FFR measurements with induced hyperaemia was 91 % in the ADenosine Vasodilator Independent Stenosis Evaluation study, which included 157 stenoses,62 80 % in a multicentre study with 392 stenoses63 and only 60 % in a multicentre study in 206 consecutive patients.64 In a large multicentre study (RESOLVE: Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pa with fractional flow reserve), pd/pa and iFR were compared to FFR in 1,768 patients.65 It was demonstrated that 0.90 was the optimum cut-off for the iFR to identify stenoses with an FFR <0.80. For pd/pa, the optimal threshold value was 0.92. There were no significant differences between the two methods of measurement at rest, and in both cases, with these thresholds, approximately 80 % of the lesions were classified correctly compared to FFR. Two recent, large multicentre trials evaluated the use of the iFR versus FFR for clinical decision-making in a randomised fashion. The ‘SWEDEHEART’ trial randomised 2,037 patients with an indication for the invasive assessment of haemodynamic relevance of a coronary lesion to use either the iFR (threshold: 0.89) or FFR (threshold: 0.80) for decision-making.66 The mean iFR was 0.91 and the mean FFR was 0.82. In the iFR arm, 53 % of all patients underwent revascularisation compared to 56.5 % in the FFR arm (p=0.11). The primary endpoint (death from any cause, myocardial infarction or unplannd revascularisation during 12 months of follow up) was not significantly different between the groups (6.7 % versus 6.1 %, p=0.53). Using the same thresholds for decision-making, another trial randomised 2,492 patients to decision-making based on iFR or FFR.67 Again, no difference in the primary endpoint was observed at the end of the 12 month follow-up period (6.8 % versus 7.0 %), but significantly fewer baseline revascularisations were performed in the iFR group (47.5 %) compared to the FFR group (53.4 %, p=0.003).
Thus, in the authors’ opinion, vasodilation can cleary be avoided in the case of resting iFR or pd/pa values that are either near 1.0 or ≤0.80. Using non-vasodilation-dependent parameters with adapted thresholds for decision-making could be a justified alternative if there were a need to avoid adenosine.
Limitations of Fractional Flow Reserve Measurements
The concept of diagnosing lesion-specific coronary ischaemia through FFR requires hyperaemia following maximum vasodilation of the coronary microvasculature through adenosine administration.68 Therefore, there is a possibility that in the presence of microvascular dysfunction, such as in the case of previous myocardial infarction,69 in the case of left ventricular hypertrophy or as a result of diabetic microangiopathy, the extent of achieved maximum hyperaemia will be less than in healthy individuals, meaning that the measured pd is incorrectly high, and potentially, FFR is false negative.70 In absolute terms, the changes in FFR in patients with microvascular perfusion impairment appear rather low (5 %, or in absolute values, 0.05).71 However, in the FFR borderline area (approximately 0.80), this can lead to misinterpretation of haemodynamic relevance, so that in patients with borderline FFR values and suspected impairment of microvascular function, particular care must be taken regarding the interpretation of measurement results. FFR measurements also yield incorrectly high values (and thus possibly a false-negative result) in patients with severe hypotension.72 Therefore, FFR cannot, for example, be used for revascularisation decisions in patients with cardiogenic shock.
Seto et al. observed unstable (i.e. increasing) pd/pa measurements in the course of continuous intravenous adenosine administration in 22 of 68 patients.73 Under maximum hyperaemia, there was a disproportionately high increase of pd, and FFR on average increased by 0.08. In 28 % of the patients examined, this caused FFR to exceed the threshold value of 0.80. This variability means that there is a risk of false-negative FFR measurements during the long-term infusion of adenosine. While the cause of the phenomenon is unclear, it might make the assessment of multiple or serial stenoses in a coronary vessel using a pullback manoeuver during extended administration of adenosine more difficult.
There are further limitations due to the fact that certain patient groups were not included or tested in the randomised FFR studies. In the FAME studies, the excluded patient groups comprised the following: patients with left main coronary artery stenosis, patients with a recent history of STEMI, patients with a history of bypass surgery, patients with impaired left ventricular (LV) systolic function (LV ejection fraction <30 %) and patients with left ventricular hypertrophy (>13 mm).
For these patient groups, there are currently no randomised, controlled outcome studies to validate FFR. Even though these limitations are not widely considered in everyday practice (particularly impaired LV function and LV hypertrophy), they should be given particular attention in the case of borderline measurement results.
Clinical Perspective
A substantial further increase in our knowledge about intracoronary pressure wire and FFR measurements can be expected in the next several years.72 Numerous ongoing, prospective studies in special patient populations will significantly increase the body of available data regarding the integration of FFR measurements into clinical decisionmaking. In addition, methodical work can be expected to further clarify the value of various drugs and routes of administration for inducing hyperaemia, and provide further data regarding the clinical reliability of pd/pa measurements without inducing hyperaemia.73
Furthermore, several methods of non-invasive FFR measurement with computational fluid dynamics are currently being evaluated; for example, based on computed tomography (CT) data,75–77 3-dimensionally reconstructed coronary angiograms78–83 and optical coherence tomography.84 However, these methods are still in the development and validation phases, and are not yet ready for clinical use. Among the methods mentioned, CT-based FFR has the most available data.
Overall, given the available data from prospective studies and the clear recommendations in all the relevant guidelines, FFR measurement should be a readily available as part of the diagnostic repertoire in all cardiac catheterisation laboratories, and physicians and support staff should be intimately familiar with the process and interpretation of FFR. Experience shows that barriers to the use of FFR are significantly reduced when all staff within a catheterisation facility are able to follow a clearly-defined routine workflow with the fewest possible number of steps to measure FFR, whether with the intravenous administration of adenosine, the intracoronary administration of adenosine or the administration of another drug. This in particular pertains to the preparation of the measuring devices and preparation of the medication to induce hyperaemia.