Women in the US are more likely to die from pregnancy- or childbirth-related causes than women in any other high-income country.1 The Centers for Disease Control and Prevention (CDC) define a pregnancy-related death as:
“The death of a woman during pregnancy or within one year of the end of pregnancy from a pregnancy complication, a chain of events initiated by pregnancy, or the aggravation of an unrelated condition by the physiologic effects of pregnancy.”2
According to the CDC, the three most common and potentially preventable pregnancy-related complications are postpartum haemorrhage, severe hypertension and venous thromboembolism.3 Cardiovascular disease is the leading cause of pregnancy-related death in the US, accounting for 25–30% of all maternal deaths, with cardiomyopathies accounting for between one-half and two-thirds of these cases.4,5 In addition, 1–2% of pregnancies are complicated by cardiac disease.6
Aetiologies
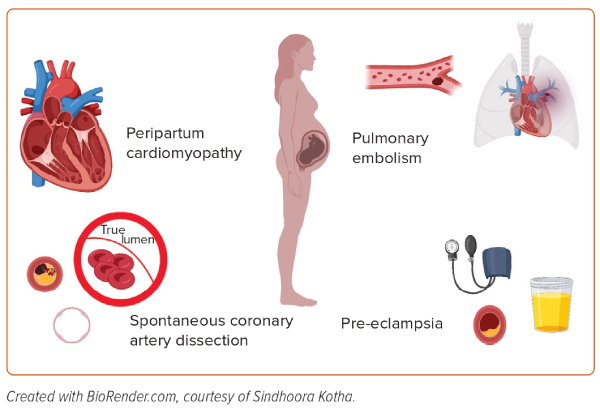
The aetiologies of heart failure in pregnant women can be grouped into four categories (Figure 1):
- the unmasking of pre-existing cardiovascular disease by pregnancy;
- an identifiable acute cardiac insult, including spontaneous coronary artery dissection (SCAD) and pulmonary embolism (PE);
- pre-eclampsia/eclampsia; and
- peripartum cardiomyopathy (PPCM).7
During pregnancy, women can commonly experience symptoms that mimic cardiovascular disease, including dyspnoea, dizziness, orthopnoea, palpitations and peripheral oedema; therefore, identifying heart failure in pregnant women can be challenging.8
The maternal cardiovascular system is adaptive during normal pregnancy to sustain the growth of the fetus, but this can be challenging in women with established cardiovascular disease. These adaptive maternal changes include a hyperdynamic circulation, vasodilatation, increased filling capacity of the vasculature and, consequently, volume expansion by approximately 40% by 24 weeks gestation, which then peaks around 30 weeks gestation.9
There is a smaller increase in the red blood cell mass in comparison, which leads to a fall in serum haemoglobin concentrations in pregnancy.10 HbA1c concentrations are also lower in normal pregnancy due to the increased red blood cell turnover.11 Furthermore, pregnancy results in a 15–25% increase in heart rate, which peaks in the third trimester and normalises 10 days postpartum to the prepregnancy state.6
Normal pregnancy also requires significant maternal cardiometabolic adaptation, with a 30–50% increase in cardiac output due to an increase in stroke volume initially; alterations in lipid profile, including an approximate 50% increase in total cholesterol; and a significant increase in insulin resistance during the second half of gestation to facilitate the transfer of glucose to the fetus.9 The state of insulin resistance encourages the breakdown of fat stores and increased endogenous glucose production.12
These changes can unmask pre-existing cardiomyopathies, valvular heart diseases and congenital heart diseases that are intolerant to the volume load. Conversely, pregnancy-associated MI, spontaneous coronary artery dissection, pulmonary thromboembolism and amniotic fluid embolism can result in a heart failure syndrome and may present with cardiogenic shock (CS); severe pre-eclampsia and eclampsia can manifest as volume overload, and PPCM may present with heart failure, secondary to idiopathic left ventricular (LV) systolic dysfunction.13,14 Most cardiovascular conditions typically present by the second trimester, whereas PPCM is more common towards the end of the pregnancy and in the months following delivery.15
Development of CS is a potentially life-threatening complication of acute cardiovascular impairments during pregnancy. In this regard, prompt recognition of CS is pivotal to maximise medical assistance and optimise outcomes. The parameters for diagnosing CS in pregnancy rely on clinical and haemodynamic criteria, including sustained systolic blood pressure <90 mmHg for 30 minutes, evidence of end-organ hypoperfusion and lactate >2 mmol/l. Haemodynamic criteria for CS include a cardiac index of <1.8 l/min/m2 without vasopressors or inotropes and cardiac power output <0.6 W.16
There is also a reduction in cardiac output, which leads to a cycle of hypoperfusion, inflammation, pulmonary congestion and ischaemia.17
Women with advanced heart failure are under-represented in trials on short-term and durable mechanical circulatory support (MCS), although they derive similar benefit.18 Moreover, intensive medical and interventional therapies are effective but often underutilised in acute heart failure.18 LV assist devices (LVADs) have expanded the possibility to use durable support in women; however, the incidence of stroke has been reported to be higher in women supported with LVAD than in men.19,20 Understanding the potential use and degree of effectiveness of temporary MCS devices may contribute to further tailoring of treatment in this complex group of patients.
Haemodynamic Phenotypes of Cardiogenic Shock and Device Selection
Devices for MCS have been widely adopted in clinical practice in the setting of CS. Systematic data regarding their use during pregnancy, especially considering the specific complications that may occur, are scarce. However, appropriate understanding of their potential applications, their effects on haemodynamics, their anatomical requirements and their limitations may effectively guide towards providing a tailored therapy for critically compromised pregnant patients.
CS can be classified according to the congestion profile and depending on the involvement of one or both ventricles. Invasive haemodynamic monitoring with pulmonary artery catheters allows for the rapid identification of underlying pathophysiological alterations. LV-dominant congestion may be suspected if the pulmonary capillary wedge pressure (PCWP) is elevated, or if the LV end-diastolic pressure is >15 mmHg. Conversely, normal PCWP but elevated right atrial pressures may suggest right ventricular-predominant congestion. Notably, combined alterations may indicate biventricular failure.21
The aetiology of CS can also contribute to the characterisation of the phenotype. PPCM is typically characterised by LV dysfunction and heart failure; therefore, LV-predominant involvement and congestion may be initially suspected. Conversely, PE tends to lead to right ventricular dysfunction with potential development of right ventricular-predominant congestion.15,22
Regarding available devices, left-sided percutaneous ventricular assist devices (pVADs), such as Impella (2.5, CP, 5.0 and 5.5; Abiomed) and intra-aortic balloon pump (IABP), contribute to the restoration of cardiac output, but they require a degree of residual LV function.21,23 Conversely, extracorporeal membrane oxygenation (ECMO) may be useful in patients with combined CS and respiratory insufficiency or refractory cardiac arrest. ECMO provides up to 7 l/min flow, and therefore allows for a better end-organ perfusion; nevertheless, in case of high flow or aortic regurgitation, it may cause severe LV distension and left side congestion, and may potentially lead to pulmonary oedema. In this regard, venting techniques, such as the ECPella combination (ECMO + Impella), may contribute to unloading the LV.23
Biventricular support options, such as ECPella and BiPella (left-sided+right-sided Impella) combinations, can be useful in case of the need for LV unloading and in the setting of biventricular failure without pulmonary failure or respiratory insufficiency, respectively.23
To date, data regarding the indications and timing for MCS implementation are scarce, and primarily rely on single-case heart team consensus.23
Access site difficulties and mechanical interference with the fetus, especially in advanced stages of pregnancy, may be concerning. Although very limited descriptions of alternative implantation methods are available, axillary implantation of Impella and the lateral decubitus position for venoarterial ECMO (VA-ECMO) have been reported for the safe placement and adequate functioning of the devices.24,25
Peripartum Spontaneous Coronary Artery Dissection and Shock
The incidence of coronary artery disease in women of child-bearing age is low, and acute MI (AMI) is uncommon.26,27 However, pregnancy has been shown to increase the risk of AMI approximately threefold compared with the risk in non-pregnant women of similar age, with SCAD being the most common cause of pregnancy-associated AMI (43%).28 SCAD is defined as an epicardial coronary artery dissection not associated with atherosclerosis or trauma and not iatrogenic; the predominant mechanism of myocardial injury occurring in SCAD is coronary artery obstruction caused by formation of an intramural haematoma or intimal disruption.29 SCAD most commonly occurs in patients with few or no traditional cardiovascular risk factors, and the clinical presentation can include unstable angina, MI, ventricular arrhythmias and sudden cardiac death. In the setting of SCAD, CS presentation is infrequent and may be related to dissection progression or disease affecting multiple vessels.30–32 In fact, SCAD mostly involves the left anterior descending artery; however, multivessel involvement has also been reported.30
Observational studies have shown that percutaneous coronary intervention for the treatment of SCAD is associated with lower technical success and higher complications, whereas conservative measures have favourable outcomes in most cases; therefore, accurate diagnosis is fundamental not only to provide early supportive care, but also to ensure that an invasive strategy is reserved for a select group of patients in extreme circumstances, such as ischaemia caused by total vessel occlusion.30,32–36
Recently, data have been extracted from the US National Readmission Database to evaluate the incidence and outcomes of SCAD, as well as the likelihood of developing CS; the results showed that SCAD patients had a higher incidence of CS and were more likely to receive MCS.37
The literature regarding the use of MCS during CS in the setting of SCAD is scarce, but the implementation of ECMO, IABP, LVAD and Impella has been reported.38–40 A recent case report described the use of Impella both as bridge-to-decision and as a postoperative support in a case of pregnancy-associated SCAD.40 Therefore, regardless of the paucity of the clinical data regarding the use of MCS during CS in the setting of pregnancy-associated SCAD, there is evidence of its efficacy; further studies are needed to understand the best implementation strategies.
Pulmonary Embolism and Shock
According to data from the World Health Organization (WHO), venous thromboembolism (VTE) is responsible for 3% of all maternal deaths worldwide.41 Pregnancy induces a prothrombotic state, characterised by an increase in coagulation factors, decreased natural anticoagulants and impairment of fibrinolysis. The hypercoagulable state that occurs during pregnancy has most likely evolved to protect women against the risk of bleeding during miscarriage and childbirth.42 Several factors can increase the risk of VTE during pregnancy, including inherited thrombophilia, antiphospholipid syndrome or a previous history of thrombosis.42–44
Compared with non-pregnant women, pregnancy increases the risk of deep vein thrombosis up to fivefold, and acute VTE events occur in 1–2 per 1,000 pregnancies.44–46 Regarding the clinical presentation, dyspnoea, tachycardia and leg swelling are common in pregnant women; therefore, typical PE symptoms are non-specific during pregnancy.47 Moreover, clinical probability scores, such as the Wells model, and Geneva criteria have not been validated in pregnant women.48–50 The treatment strategy is complex, because it should balance efficacy with fetal safety, teratogenicity and pharmacodynamics, and weigh the risk of anticoagulation with that of clotting recurrence.51 In haemodynamically stable patients, immediate treatment consists of anticoagulants as the first-line treatment: low molecular weight heparin and unfractionated heparin can both be used safely in pregnancy because they do not cross the placental barrier. Clinical trials for direct/novel oral anticoagulants excluded pregnant women, whereas warfarin crosses the placenta and is teratogenic.51,52
Pregnant women who are haemodynamically unstable or severely hypoxaemic should be treated with thrombolysis if there are no contraindications; however, data on managing pregnant patients in shock from VTE with other interventions besides systemic anticoagulation are limited.51 There are few case reports documenting the administration of thrombolytics to pregnant patients with no resulting complications for the mother or the newborn, and the best results have been obtained with tissue plasminogen activator.53 Maternal bleeding is the main risk of thrombolysis, and has been reported in 8% of treated patients; conversely, the cause of fetal death is more complex to adjudicate, and may be equally relatable to thrombolytics or to the haemodynamic instability induced by the PE.51,54 Catheter-based thrombolytic administration may have lower complication rates due to the lower medication dosages required; however, its use in pregnancy is supported by the description of only a few cases.55.
Implementation of MCS has been described among other salvage therapies in pregnant patients with haemodynamically unstable PE, aiming at avoiding systemic thrombolysis and therefore reducing the higher risk of severe bleeding in the immediate postpartum period. A recent systematic review included 127 cases of severe PE during pregnancy and until 6 weeks postpartum treated with thrombolysis, thrombectomy and/or ECMO. The latter was used in 14 patients, three of whom received support with ECMO and anticoagulation only; overall reported survival rates were 94%, 86% and 100% following thrombolysis, surgical thrombectomy and sole ECMO support, respectively.56
Notably, use of the ECPella combination has been reported in the management of amniotic fluid embolism complicated by CS and cardiac arrest, leading to safe delivery and maternal recovery.57,58
Data regarding the use of pVADs in peripartum PE are very scarce; however, the feasibility and efficacy of support with Impella RP (right-sided Impella) in PE-induced right ventricular failure is well established in the literature.59–64 In this regard, more research would be needed to define the role of MCS and pVADs in peripartum PE.
Peripartum Cardiomyopathy and Shock
PPCM is a severe and potentially life-threatening pregnancy-associated disease, which occurs mostly in the peripartum period and is marked by LV dysfunction and heart failure.15 The definition also includes takotsubo cardiomyopathy, which follows a different pathophysiological mechanism and can be diagnosed through echocardiographic criteria.65 The aetiology of PPCM remains unknown, but many potential causes have been hypothesised, including viral myocarditis, nutritional deficiencies, autoimmunity, microchimerism and haemodynamic stresses.66 Overall, PPCM is likely to be a multifactorial process, involving predisposing genetic mutations and environmental factors, increased oxidative stress, impaired microvasculature and inflammation.67
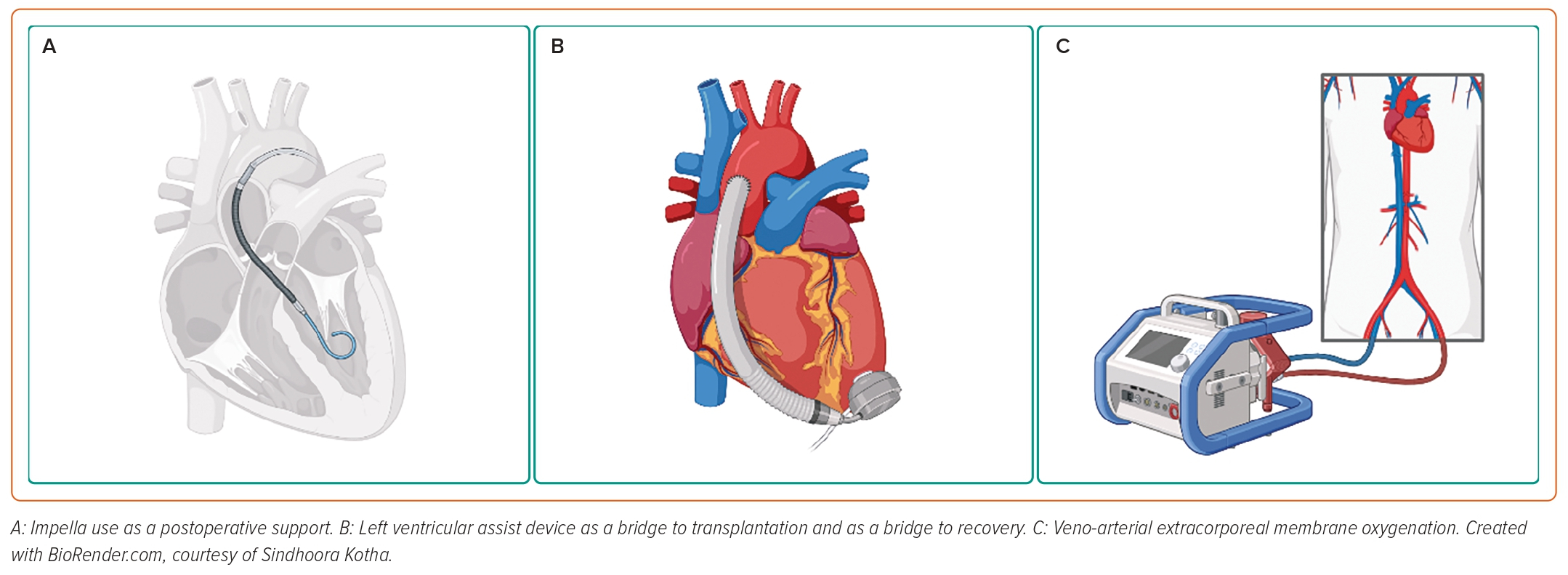
Although the treatment of most pregnancy-related complications has improved, PPCM increasingly contributes to mortality, because the need for more specific treatment algorithms remains unmet; in a population study of maternal cardiovascular deaths in California between 2002 and 2006, PPCM was the leading cause, accounting for 23% of events.68 However, improved survival rates in recent years emphasise the importance of the early recognition and initiation of heart failure treatment. Inotropes, IABPs, LVADs, biventricular assist devices and ECMO have been used successfully in these cases (Figure 2).69–73 Consistently throughout the available literature, CS patients who are refractory to medical management are successfully bridged to recovery on extracorporeal circulatory devices or survive with a long-lasting implantable ventricular assist device.
The use of LVADs for refractory CS in PPCM has been described quite extensively, both as a bridge to transplantation and as a bridge to recovery, as well as final therapeutic option.74–77 Interestingly, weaning was possible in up to one-third of treated women, which allows us to speculate that temporary MCS may be beneficial in select cases.75
Many case reports have described IABP as being instrumental in supporting women with PPCM until native LV recovery or as a bridge to another MCS device, to ease weaning from other MCS such as ECMO, and to support haemodynamically unstable patients during surgical delivery.72,78,79
VA-ECMO alone or in combination with IABP has been used successfully in patients with PPCM who develop refractory CS; unexpected vaginal bleeding was reported as a recurring complication; however, this was safely managed and did not have a significant clinical impact.80,81 Indeed, major bleedings are a significant limitation to ECMO in postpartum patients, with a reported incidence of 30–60%.82 Survival rates are promising despite the deteriorated clinical picture; however, avoiding complications is pivotal to achieving acceptable mortality profiles.82,83
Several case reports and case series describe the implementation of Impella in the setting of PPCM, in combination with bromocriptine, inotropes or ECMO.24,84–86 All data convey concordant outcomes on reversion of acute conditions, such as severely reduced ejection fraction and functional mitral regurgitation due to acute atrial dilatation, as well as high survival rates.84–86 Remarkably, early LV support with microaxial flow pumps resulted in a better myocardial recovery compared with delayed Impella implantation.86
Overall, CS in PPCM is characterised by discrete potential of recovery and may benefit from various support devices if refractory to medical therapy only, and the advent of pVAD is a promising option for management of CS during pregnancy. The development of large registries of patients, such as the EURObservational Research Program international PPCM registry, will provide fundamental data and promote further investigation of this condition.87
Conclusion
CS can occur during pregnancy and markedly worsens peripartum outcomes. However, improved survival rates in recent years emphasise the importance of the early recognition and initiation of heart failure treatment, which is being progressively implemented with novel MCS devices, specifically pVADs. The outcomes achieved with such devices are promising, but data on systematic use are scarce and insufficient to formulate indications for implementation. From our perspective, the development of large registries of patients could provide the variety of cases needed to develop consensus on the management of peripartum refractory CS.