Transcatheter aortic valve replacement (TAVR) has emerged as the treatment of choice among inoperable patients with symptomatic severe aortic stenosis and as a treatment alternative to surgical aortic valve replacement (SAVR) for high-risk surgical patients1–3. Subsequently, the number of patients undergoing TAVR worldwide is steadily increasing and the complications related to valve implantation have been well-recognised.
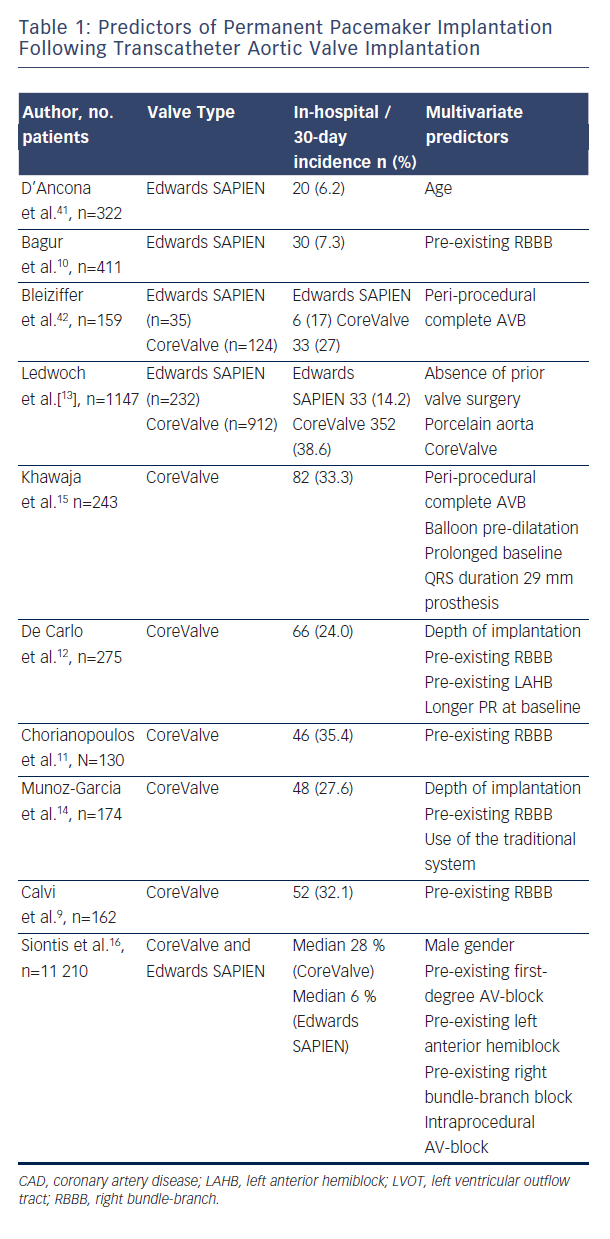
Conduction disturbances after SAVR have been extensively documented and are in part predicted by pre-existing conduction abnormalities4. According to recent data, PPM implantation is necessary in approximately five per cent of patients undergoing SAVR. Among transcatheter heart valves (THV), rates of conduction abnormalities vary from less than 10 % to more than 50 %. Depending on the reported data referred to, historical data showed that up to one-third of patients required implantation of a PPM following TAVR5. Since the debate about predictors for pacemaker implantation and their impact on outcome after TAVR is still ongoing, this review will assess the current status quo.
Predictors of PPM Implantation
Pre-existing Conduction Abnormalities and
Anatomical Conditions
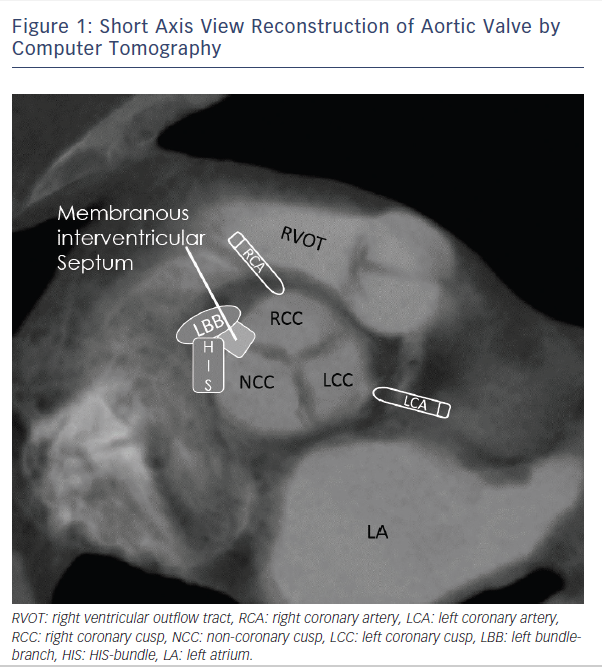
Patients undergoing TAVR have similar rates of pre-existing conduction disease as SAVR patients, which are described at 40–50 % in both surgical and transcatheter populations4,6. In an early study with the self-expanding Medtronic CoreValve Prosthesis (MCP, Medtronic, Minneapolis, Minnesota), left bundle-branch block (LBBB) at baseline, increased interventricular septal diameter (>17 mm) and increased non-coronary aortic cusp thickness (>8 mm) were highly predictive for PPM (receiver operating characteristic area 0.93±0.055, P< 0.001)7. In this analysis, non-coronary aortic cusp thickness was the strongest predictor (P=0.002, correlation coefficient =0.655). A similar study by Baan et al.8 found in 34 MCP patients that small left-ventricular outflow tract diameter, left axis deviation, significant mitral annular calcification and lower post-implant valve area are predicting post-TAVR PPM. Several studies showed that right bundle-branch block (RBBB) at baseline is one of the most significant predictors of PPM after TAVR9–14. Additionally, baseline first-degree atrioventricular (AV) block10, left anterior hemiblock12 and intraprocedural AV block15 are important predictors for PPM dependence. Most of the previously mentioned studies looked at a low number of cases. In contrast to the recent meta-analysis of Siontis et al., RBBB (n=2158; risk ratio (RR): 2.89 (CI: 2.36–2.54), p<0.01), baseline AV block (n=1381; RR: 1.52 (CI: 1.15–2.01), p<0.01), and left anterior hemiblock (n=1065; RR: 1.62 (CI: 1.17–2.25), p<0.01) were the strongest predisposing conduction disturbances for PPM16. Figure 1 emphasises the close anatomical relationship of the cardiac conduction system referring to the aortic valve and highlights the importance of pre-existing conduction abnormalities as a predictor for PPM after TAVR.
Prosthesis Type and Time of PPM
Prosthesis Type
The incidence of AV conduction disturbances as a result of TAVR and the subsequent requirement for permanent pacing differs between the two most widely used bioprostheses, the balloon-expandable Edwards SAPIEN valve (ESV) (Edwards Lifesciences, Irvine, California) and the self-expanding MCP. PPM implantation has been reported with a rate between 5–14.2 % [2,13,17–19] for the former Edwards valves (Edwards SAPIEN and Edwards SAPIEN XT) and 13.3 % for the new Edwards SAPIEN 3 THV20, whereas the need for PPM has been higher with use of the MCP (up to 24 % in the FRANCE-2 [French Aortic National CoreValve and Edwards] registry and 33 % in the UK CoreValve registry)21,15.
Early data showed a more liberal pacemaker implantation strategy after MCP TAVR and resulted in higher PPM rates, whereas newer data of the Advance II trial, which were presented at EuroPCR 2014 (ADVANCE II: Low incidence of permanent pacemaker for self -expandable valve by Petronio A. S. et al. at Euro PCR 2014, May 22, Paris, France) show that PPM rate was reduced to 13.3 % at 30 days’ follow up when MCP was deployed according to best recommendation practice (implantation depths <6 mm). Recent data of the CoreValve Extreme Risk pivotal trial revealed a PPM implantation rate of 21.6 % at 30 days22.
In addition to the above-mentioned THVs, four so-called next-generation devices have been evaluated in recent trials. The multicentre non-randomised DISCOVER trial (100 patients) recently assessed the outcomes of the non-metallic direct-flow medical THV in surgical high-risk patients. The device is fully repositionable and retrievable until polymer exchange. In DISCOVER, the overall PPM rate at 30 days was 17 %23.
The REPRISE studies assessed the outcomes with the new mechanically expanding LOTUS valve, which can be fully retrieved, redeployed or repositioned. The 30-day results of the REPRISE II trial (120 patients), which was a prospective, single-arm, multicentre study showed a PPM rate of 28.6 % at 30 days24, which might be explained by the fact that all patients with borderline aortic annulus had to undergo TAVR with the larger 27 mm prosthesis, since the 25 mm prosthesis was not available at that time.
Thirty-day outcomes from the multicentre European pivotal trial for transapical transcatheter aortic valve implantation with the self-expanding Medtronic engager valve prosthesis (Medtronic, Inc., Minneapolis, MN, USA), which consists of a self-expanding nitinol frame and a bovine pericardial tissue valve, revealed a 30-day PPM rate of 26 %25.
The transapical JenaValve™ THV system showed a 30-day pacemaker implantation rate of 9.1 %26. Other new transcatheter valves are currently under development, and will be evaluated in the near future.
Persistence of Conduction Abnormalities and time to Pacemaker Implantation:
Persistence of conduction disturbances and high-degree AV block over time is not yet clear, and seems to differ between MCP and ESV. Houthuizen reports approximately 40 % of patients developed a new LBBB after TAVI; most of these persisted at follow-up, but MCP had a twofold lower tendency to resolve27. A new LBBB occurs
2.5 times more often after MCP than after ESV implantation and is also associated with reduced recovery28. In another MCP analysis, a proportion of AV conduction disturbances after the intervention has been shown to recover over time at three months of follow-up, and only 40 % of the PPM patients for high-degree AVB still had an AVB underlying their paced rhythm29. Due to the relatively low sample size of these studies, this issue needs further investigation.
Data in relation to the appropriate time point of pacemaker implantation are rare. Simms et al. published a cohort of 100 TAVR patients who received the MCP. Average time to pacemaker implantation was four-and-a-half days (confidence interval: 2.8–6.2)30. In another cohort with the MCP prosthesis (n=270), median time to implantation of a pacemaker was four days (interquartile range [IQR], 2.0 to 7.75 days)15. Bagur et al (n=411) reported that PPM was performed at a median of two days (IQR: 0–4 days) after TAVR with ESV10.
The exact time point for PPM implantation is still an ongoing debate. Another aspect, which has to be kept in mind, is the fact that self-expanding prostheses may lead to delayed injuries of the conduction system. So far, there is no explicit data for the best time point for PPM implantation in patients after Boston Lotus, Medtronic engager or Jena valve implantation.
Pre- and Post-dilatation and Prosthesis Sizing
The close relationship of the conduction system to the aortic annulus may lead to a mechanical interaction between the prosthesis stent frame of the transcatheter valve prosthesis and the left bundle-branch, which in turn may translate into the occurrence of an LBBB and eventually into a higher grade or complete atrio-ventricular block. A study by Lange et al.31 analysed the impact of valvuloplasty balloon catheter size on the need for PPM in a larger cohort of 237 patients without prior pacemaker, who underwent TAVR with the MCP. In this analysis, the overall incidence of PPM was 21.1 %, but was significantly higher when a 25 mm balloon was used (27.1 %) than when a 23 mm
or smaller balloon was used (15.4 %) for the balloon valvuloplasty (BAV). When stratified by THV size (26 or 29 mm), there was still a stepwise increase in PPM rate with each increase in balloon size. This association of balloon size with need for a PPM remained significant after multivariable adjustment for baseline patient characteristics. These results suggest that pacemaker rates after TAVR may be reduced by using undersized BAV balloons or even avoidance of pre-dilation. Two randomised studies are currently ongoing to investigate direct TAVR without pre-dilatation with the MCP (SIMPLIFY TAVI Trial; NCT01539746) and the ESV (EASE-IT Trial; NCT02127580). Interestingly, in another study post-dilation after MCP, implantation had no effect on the requirement for PPM. The reason for this observation might be the relatively short time period when the aortic annulus is exposed to high pressure from repeated valvuloplasty31. Post-dilatation after ESV TAVR ranges between 20–41 %32–34 and shows no impact on PPM rate. A recent analysis showed a low post-dilatation rate of 4 % after Edwards SAPIEN 3 TAVR33.
The degree of prosthesis oversizing may lead to a higher incidence of PPM. Schroeter et al. found larger or significantly oversized prostheses to be an independent risk factor for PPM implantation following TAVR with the MCP35. In contrast, a study of Binder et al. with 89 patients receiving a SAPIEN XT THV showed that annular area oversizing was not associated with new conduction disturbances and permanent pacemaker implantation36. If this difference can be explained by the fact that the ESV prosthesis is oversized to a lesser degree to prevent annulus rupture remains unclear and needs further investigation.
Implantation Depths and Approach
Many studies have shown that the CoreValve prosthesis implantation depth is a predictor for PPM. The deeper the CoreValve frame protrudes into the left ventricular outflow tract, the more likely the patient is to develop an LBBB. In an early study by Piazza et al.37,
the mean implantation depth was 10.3 mm in those patients with new LBBB versus 5.5 mm in those without LBBB. Another study proposed a cutoff of 6.0 mm as an independent predictor of the development of a high-degree AV block and the requirement for permanent pacing29. This finding was confirmed for the MCP prosthesis by several other recently published studies8,38,39,12. Additionally, implantation of balloon-expandable transfemoral THVs with increased implantation depth is associated with clinically significant new conduction disturbances and permanent pacemaker implantation36. These effects also apply to ESV implantation via a transapical approach40.
The PPM incidence regarding TAVR approach is difficult to assess because patient population and risk profile are often different. A recent meta-analysis of Siontis et al.16 suggested a trend towards lower risk of PPM after MCP TAVR with the transfemoral approach compared with the transsubclavian approach (p=0.07). The same meta-analysis found no difference in PPM risk after ESV TAVR depending on the approach (transapical versus transfemoral; n=2136; risk ratio:
0.89, 95 % CI: 0.64-1.25; p=0.89).
Cost and Outcome of PPM Implantation
Cost
Patients with AV conduction disturbances after TAVR are disposed to prolonged hospitalisation and use of in-hospital continuous telemetry43, both of which result in a considerable increase of
overall cost of the TAVR procedure. Data from the FRANCE registry found that receiving a pacemaker was associated with a 36 % increase in cost44. Gutmann et al. assessed elevated procedural costs of €1,946 in case of PPM implantation in a German healthcare analysis45. Compared with surgical aortic valve replacement (SAVR), in-hospital costs were higher in TAVR patients than in SAVR patients (€40,802 versus €33,354, respectively; p=0.010)46). Reducing PPM
rate would have an impact on the cost-effectiveness of TAVR relative to that of SAVR.
Outcome
The impact of new-onset LBBB, with or without need for PPM implantation, on patient outcome after TAVR is still under debate. Houthuizen et al. found negative effects of a procedure-related LBBB on survival in a group of 679 patients after TAVR. They described an all-cause mortality of 37.8 % (n=88) in 233 patients with new-onset LBBB and 24.0 % (n=107) in patients without LBBB (p=0.002) after a median follow-up of 450 days. Consecutively, a procedure-related LBBB was an independent predictor for all-cause mortality (hazard ratio: 1.54; confidence interval (CI): 1.12–2.10) in this cohort47.
Schymik et al. confirmed that a persistent new-onset LBBB was associated with increased mortality in a cohort of 634 patients who were treated by either an MCP or ESV prosthesis. The one-year all-cause mortality rate was also higher in patients with persistent new-onset LBBB (20.8 %, n=41) than in patients without LBBB (13.0 %, n=57; p=0.010). Multivariate regression analysis revealed again that persistent new-onset LBBB was an independent predictor of all-cause mortality at one year (HR 1.84, 95 % CI 1.35–2.02).
However, Buellesfeld et al. recently published data on 353 patients undergoing TAVR with the CoreValve prosthesis and found that PPM implantation had no impact on major adverse cardiac and cerebrovascular events or mortality outcome one year after TAVR48. Other study groups found no difference in mortality regarding LBBB or PPM49–51. Hoffmann et al. found a significantly higher left ventricular ejection fraction in patients after TAVR without LBBB (59±10 %) as compared with those with LBBB during 12 months of follow-up (51±12 %; p=0.052), but found no difference in clinical outcome52. These findings were confirmed by Nazif et al. who presented the one-year results of the PARTNER trial and focused on new-onset LBBB which was not associated with significant differences in
one-year mortality, cardiovascular mortality or repeat hospitalisation. However, it was associated with increased PPM implantation
during hospitalisation (8.3 % versus 2.8 %, p=0.005) and the ejection fraction failed to improve after TAVR in patients with new LBBB and remained lower at six months to one year (52.8 % versus 58.1 %, p=0.001)53. The independent predictors of the outcome in patients with new-onset LBBB (including the protective or detrimental effect of pacemaker implantation after TAVR) are currently unclear and still need further investigation.
Clinical Implication
Temporarily inserted pacing leads are mandatory for rapid pacing during the procedure as well for at least 24 hours after TAVR for therapy of delayed bradycardia. Patients with self-expanding valves like the MCP should be monitored for at least 48 hours after TAVR54. In case of new-onset LBBB or AV block, ECG monitoring should be continued for five days. In particular, those patients with
pre-existing conduction disturbances such as RBBB or first-degree AV block should be carefully monitored with daily ECG. Pacemaker rates after TAVR may be safely decreased by avoiding pre-dilatation or use of undersized balloons31, as well as correct positioning
of the prosthesis37.
Future Considerations
Increasing data from observational studies involving new valve technologies, such as the Direct Flow Medical (Santa Rosa, CA, USA), the Lotus Aortic Valve prosthesis (Boston Scientific Corporation, Marlborough, Massachusetts), the JenaValve™ system (JenaValve Technology
GmbH, Munich, Germany), the transfemoral Medtronic Evolut R,
and the transapical Medtronic Engager (Medtronic, Minneapolis, Minnesota) show promising results and improve outcomes by minimising
procedure-related complications. Future studies might give new answers on predictors and outcome after TAVR regarding PPM.
Conclusion
Despite being less invasive than open SAVR, TAVR remains associated with potential procedure-related complications. New LBBB and the need for PPM implantation are the most frequent adverse events after TAVR. The incidence of significant conduction disturbances is dependent on the TAVR prosthesis used and has decreased as a result of improved implantation techniques. In addition, next-generation devices with reduced interaction with the LVOT might further decrease conduction disturbances after TAVR. Minimising PPM rate is important, especially as TAVR technology could be increasingly applied to younger and healthier patients.