Evidence-based medicine is the foundation of contemporary clinical practice and results in better clinical outcomes than experience-based medicine.1 Meta-analyses of homogenous randomised controlled clinical trials are the pinnacle of evidence-based medicine and the backbone of the highest recommendations in clinical guidelines.
These randomised trials pertain only to the selected patients who meet the predefined inclusion/exclusion criteria but are applied as a one-size-fits-all approach in guidelines. Medical advances are rapidly continuing, with a plethora of medical and device concepts emerging for any given condition in any given patient becoming hard to capture in formal treatment guidelines. Furthermore, patient preference and shared decision-making have recently gained a higher profile.
Precision medicine is the new paradigm and is focused on the needs of an individual patient. It was recently defined as “treatments targeted to the needs of an individual patient on the basis of genetic, biomarker, phenotypic or psychosocial characteristics that distinguish a given patient from another patient with similar clinical presentation.”2,3 Computational modelling may assist precision medicine by integrating individual patient data (the phenotype) to stratify risk and potentially identify more precise therapeutic solutions and simulate the effects of a therapy in the individual person of interest.2,3 In short, the paradigm is shifting from the average to the individual person of interest.4
Precision Medicine in Practice
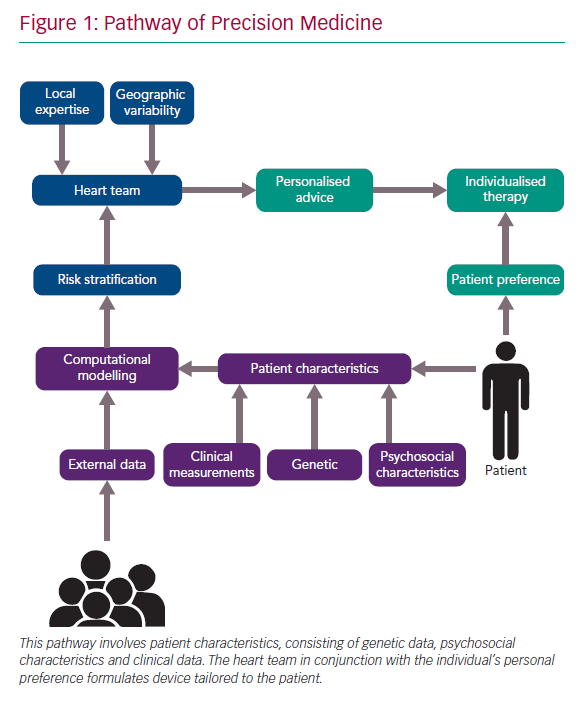
Precision medicine relies on biological, mechanical and personal variables to optimise individual therapy (Figure 1). Examples of precision medicine in interventional cardiology are the multidisciplinary heart team, the systematic use of intravascular imaging for left main stem stenting and plaque modification technology. The heart team is a tool to integrate multiple perspectives from different disciplines that are involved in the management of a patient.
The consensus of the heart team is personalised and therefore specific to the individual patient, but may vary from one heart team to another. Heart team decision-making reflects geographical variability and local institutional expertise. Some institutions may favour a surgical approach, while others may be oriented more towards interventional cardiology. More recently, the value of patient preference was added to the mix and may further determine treatment strategy selection and complement precision medicine.
Interventionists have a wide array of tools and techniques at their disposal and need to figure out their optimal implementation to justify financial cost, procedural time and clinical benefit. Arguably, systematic use of intravascular imaging would make more sense in left main percutaneous coronary intervention (PCI) than in a type A lesion in the mid segment of a right coronary artery. A more specific example is plaque modification of calcified coronary lesions. Rotational and orbital atherectomy, Shockwave intravascular lithotripsy (Shockwave Medical) or an arsenal of compliant, semi-compliant and high-pressure balloons can be used for this purpose. Specific plaque characteristics can mean one technology is favoured over another.
Additional intravascular imaging with intravascular ultrasound (IVUS) or optical coherence tomography optimises clinical outcomes. IVUS assesses plaque composition and distribution before PCI and identifies abnormalities such as underexpansion, malposition or edge dissections after PCI.5 These quantitative and qualitative characteristics may guide targeted, patient-tailored device selection and result in optimal lesion preparation and stent deployment with proper expansion and apposition.
Future of Computational Modelling
Through sophisticated algorithms, computational modelling allows a virtual reality representation to be created so clinicians can appreciate and simulate therapeutic strategies that relate uniquely to one particular patient. The rapid development of computational modelling may provide new possibilities to predict the risk of developing certain diseases, test new therapeutic treatments, improve medical device safety and select the best therapeutic options for individual patients.6
In silico testing, where computational modelling and artificial intelligence are combined, is an interesting new development. The Virtual Physiological Human (VPH) project is an example of this concept and started in 2005 with three objectives: introduce patient-specific modelling to support medical decision; apply in silico clinical trials to test new treatments and duplicate the robustness of clinical trials with large samples in a virtual clinical trial (its main objective); and introduce patient-specific, real-time simulations to devise tailored treatment for the individual patient.6,7
In the in silico test environment, approximately nine of 10 novel drugs entering Phase I clinical trials seem to fail. The beauty of in silico trials is that they are able to evaluate positive effects and drug toxicity precluding animal testing and reducing cost and time.8 VPH models can incorporate numerous patient-specific variables, such as lifestyle, medical history, physical examination, diagnostic tests and genetics to make reliable predictions.2 VPH might be used to predict the risk of developing certain conditions and determine which treatment should be used and when it should be started to prevent diseases on an individual level.
There are numerous challenges to implementing these models in the medical practice. Before making a patient-specific model that works in clinical practice, several issues need to be addressed. First are the granularity and type of data to be used. Computational modelling can process huge numbers of variables and irrelevant variables may camouflage underlying relationships and pollute the model. The use of existing knowledge of relevant variables based on evidence-based research should guide this selection.2,4 Second is the validation of patient-specific models before implementation in clinical practice. This validation process requires the model be tested in a properly sized patient sample.4 Machine learning, big data and artificial intelligence may help to optimise these processes.6
The VPH approach requires pathophysiological processes to be described in quantitative terms. In the first 10 years of the VPH project, the most popular targets were organ systems with clear biophysical characterisations, such as the cardiovascular system. This focus led to developments in computational modelling that may catalyse precision medicine.
Two examples of the application of computational modelling in contemporary interventional cardiology derived from in silico trials are HeartFlow FFRCT (HeartFlow) and FEops HEARTguide (FEops).
HeartFlow FFRCT generates a person-specific 3D model of the coronary arteries from static coronary CT images and simulates pressure, velocity and blood flow to predict the fractional flow reserve. With this technique, it becomes possible to determine coronary physiology and thus the functional importance of a particular stenosis in the coronary arterial tree. Computational modelling is used to compare a patient-unique CT scan with a database of CT scans to determine the clinical importance of the stenosis and thereby show non-invasively whether PCI would be effective.9 With further iterations, prediction of the effect of coronary stenting, including residual coronary flow after PCI, should be possible. This technique may also allow patients with vulnerable plaques to be identified, in whom PCI might have prophylactic benefit.10
The FEops HEARTguide integrates CT imaging with tissue and device characteristics to simulate device-host interactions and predict calcium displacement, device deformity, residual periprosthetic leak and occurrence of conduction abnormalities secondary to focal pressure phenomena in patients who undergo transcatheter aortic valve implantation for severe aortic stenosis.11,12 Computational modelling may help to identify and select the best device for any specific anatomy whether it is in the coronary or structural heart space. These technologies may prove invaluable for patient-tailored device selection and treatment in future.
Conclusion
Precision medicine reconciles evidence-based medicine with the growing armamentarium of medical options and technologies. As randomised trials remain the pinnacle of evidence-based medicine and backbone of contemporary clinical practice, physicians need to figure out how to implement the best clinical option for each individual patient. Precision medicine is being increasingly adopted in contemporary clinical practice, but has numerous layers. Further refinement by advanced computational modelling in concert with artificial intelligence and computer learning will be a prelude to the medicine of the future.