Obstructive coronary atherosclerosis and its complications (e.g. coronary thrombosis) are considered to be the most common causes of myocardial ischaemia. However, up to 50 % of stable angina patients undergoing diagnostic coronary angiography and 10–15 % of those presenting with acute coronary syndrome (ACS) are found to have normal or ‘near-normal’ coronary arteries.1,2 A large body of data indicates that coronary microvascular dysfunction (CMVD) plays an important role in causing myocardial ischaemia in these patients with angina pectoris, despite no obstructive coronary artery disease (CAD). CMVD can be found in many cardiac or systemic diseases, e.g. cardiomyopathies, coronary atherosclerosis, immunological conditions, systemic hypertension. In these patients, CMVD and myocardial ischaemia can result from mechanisms directly related to the underlying disease. In many patients, however, CMVD is the only or the prevailing identifiable mechanism responsible for the occurrence of angina episodes and myocardial ischaemia, a condition defined as ‘primary’ microvascular angina (MVA).3 The term ‘microvascular angina’ was initially proposed by Cannon and Epstein in 19888 to identify patients with myocardial ischaemia triggered not by obstructive CAD but by functional microvascular abnormalities. More recently, the COVADIS group has proposed diagnostic criteria to define MVA.4
In clinical practice, a coronary microvascular origin of typical chest pain symptoms is usually suspected, by exclusion, in patients with angina, typical ischaemic changes on the ECG and/or abnormal findings on non-invasive imaging stress tests, in whom coronary arteriography fails to show obstructive CAD or epicardial coronary artery spasm. This review briefly addresses the clinical presentation, mechanisms and management of patients with chronic primary stable MVA.
Clinical Presentation and Clues for Identifying MVA Patients
Primary stable MVA is characterised by angina episodes that are exclusively or predominantly triggered by effort or other conditions that increase myocardial oxygen demand. The ECG taken during effort usually shows typical ST-segment depression in most patients, and reversible stress-induced myocardial perfusion defects are usually detectable in over 50 % of patients.5 Of note, at variance with patients with obstructive CAD, conventional stress echocardiography less often shows left ventricular (LV) contractile abnormalities in MVA patients.6 This can be explained by the ‘patchy’ rather than regional nature of CMVD resulting in sparse myocardial perfusion abnormalities, usually affecting thin layers of the myocardium, in contrast with the involvement of large myocardial areas in stress-induced ischaemia caused by flow-limiting epicardial stenoses.7
In MVA patients exercise- and/or stress-induced angina, tends to be longer lasting with a slower resolution (>10–15 min) of chest pain after stopping exercise, and/or following the administration of short-acting nitrates, compared with anginal episodes in CAD patients.8–10 These findings are particularly suggestive of MVA when occurring in peri- or post-menopausal women. Female gender is largely prevalent among patients with primary stable MVA, a finding that has suggested a role for oestrogen deficiency in the pathogenesis of MVA in women.11 When positive for myocardial ischaemia, an ECG exercise stress test is usually unhelpful to distinguish between patients with obstructive CAD versus CMVD. In some MVA patients, both the early appearance of ECG abnormalities and/or angina during the stress test and the lack of response to sublingual nitrate administration often suggests a microvascular origin of symptoms. Myocardial perfusion scintigraphy stress testing is often unhelpful for identifying patients with angina related to obstructive CAD versus CMVD. A negative perfusion test or the occurrence of patchy perfusion abnormalities in the presence of typical effort-induced angina may suggest MVA rather than obstructive CAD, but negative findings sometimes occur in the presence of multivessel obstructive CAD. As mentioned above, the occurrence of angina and ST-segment depression, but not LV contractile abnormalities, during echocardiographic dipyridamole or dobutamine stress testing, is suggestive of a microvascular origin of symptoms.12,13 Newer echocardiographic modalities, however, are nowadays more able to detect LV contractile abnormalities in MVA patients.
Assessment of Coronary Microvascular Function
A definitive diagnosis of MVA, as also proposed by the COVADIS group,4 requires the demonstration of CMVD. Coronary microvascular function can be examined by both invasive and non-invasive methods.14 The most widely used invasive method for the evaluation of coronary microvascular function is the recording of coronary blood flow velocity using an intracoronary Doppler wire coupled with a pressure/thermodilution device to allow measurements of both blood flow and coronary microvascular resistance. Among the most reliable and accurate methods for non-invasive assessment of CMVD is positron emission tomography (PET).15 However, its reduced availability in clinical practice and high cost preclude a wider applicability for routine assessment of MVA patients. Cardiovascular magnetic resonance (CMR) imaging, with gadolinium as a flow tracer, is also a very promising tool for the non-invasive assessment of CMVD.16,17 Contrast stress echocardiography is another valuable method for the assessment of CMVD in different myocardial territories;18 it is more widely available and less expensive than other methods for coronary blood flow (CBF) assessment. Although more work is required to fine tune this technique, transthoracic echocardiographic Doppler recording (TTDE), has been shown to be a reliable and accurate methodology.14 This technique, however, is operator-dependent, and limitations also include suboptimal chest windows in some patients and interobserver variability.
When assessed, CMVD tests should explore both vasodilator and vasoconstrictor responses of the coronary microcirculation (Figure 1). Coronary microvascular dilator function is investigated by measuring coronary blood flow changes and/or resistance in response to vasodilator stimuli, i.e. adenosine injection, and to constrictor stimuli such as ergonovine or acetylcholine (ACh). Importantly, both endothelium-independent and endothelium-dependent coronary microvascular dilatation should be assessed.
Maximal endothelium-independent microvascular dilatation and flow increase (coronary flow reserve, CFR) is usually obtained by intravenous administration of adenosine (0.14 µg/kg/min) or dipyridamole (0.84 mg/kg in 6 min). A CFR <2.0 is considered diagnostic for the presence of CMVD, with values of >2.0 but <2.5 being of borderline diagnostic significance.14
Intracoronary ACh administration – at increasing doses – and the cold pressor test are used to assess endothelium-dependent coronary microvascular dilatation. These tests, however, might not induce an adequate increase of CBF because they can cause coronary microvascular constriction, resulting from a direct (ACh) or sympathetically-mediated (cold pressor test) effect on vascular smooth muscle cells. Intracoronary administration of ACh (at increasing doses of up to 200 µg/min) is at present the preferred test to assess the presence of coronary microvascular constriction/spasm in MVA patients. The induction of coronary microvascular constriction might be documented by a reduction of CBF in the absence of flow-limiting epicardial constriction/spasm. Alternatively, an ACh test may be considered positive for coronary microvascular spasm when it triggers typical angina and ischaemic ECG changes in the absence of major epicardial constriction/spasm at angiography.19
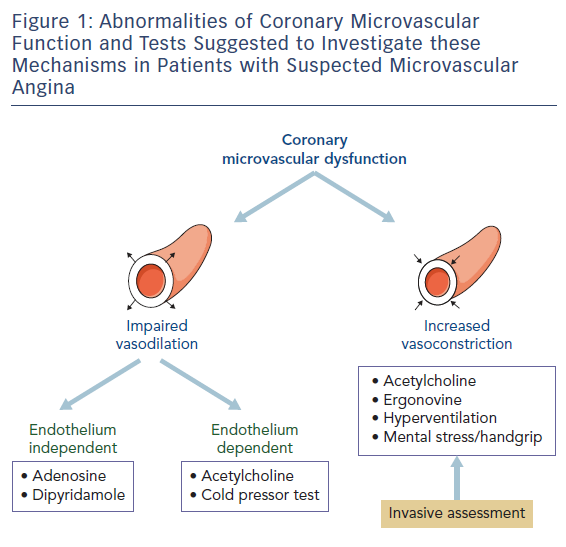
Of importance, coronary microvascular spasm can only be assessed during coronary angiography. While ACh is currently the preferred provocative stimulus for coronary microvascular spasm, ergonovine might be a valid alternative (Figure 1).
Evidence for a Role of CMVD in the Pathogenesis of MVA
Several studies have demonstrated CMD in patients with stable angina who have normal or near normal epicardial coronary arteries.20–24 Structural abnormalities of the small coronary arteries have been described in several studies, including vascular smooth muscle hypertrophy, capillary rarefaction or vascular wall fibrosis.20,21 Functional changes of the coronary microcirculation appear to be more frequently found to be the mechanism for MVA and include both a reduction of the vasodilator response and increased vasoconstrictor activity, i.e. microvascular spasm.22–24 Initial studies showed an impaired increase of coronary blood flow in response to dilator stimuli such as dipyridamole and atrial pacing using the invasive xenon wash-out and thermodilution methods, respectively.20–25 Impaired CFR was subsequently confirmed in many studies using various other methods. PET and CMR studies showed abnormal CBF responses and myocardial perfusion abnormalities involving mainly the subendocardium in patients without obstructive CAD, suggesting an involvement of the coronary microcirculation.16 Moreover, using intracoronary Doppler devices, studies in patients with MVA have documented impaired coronary microvascular dilation in response to ACh, a marker of impaired endothelium-dependent vasodilator responses, likely due to a reduced nitric oxide (NO) production by endothelial cells.26
Other studies have shown abnormal constrictor responses of the coronary microcirculation in primary MVA patients, i.e. coronary microvascular spasm.19,27–28 In the 1980s Cannon and Epstein showed for the first time that the administration of ergonovine further impaired the abnormal coronary blood flow responses to atrial pacing in patients with angina despite angiographically normal coronary arteries.25 They proposed the term ‘microvascular angina’ to define these patients.
In clinical practice, markers of a possible role of coronary microvascular constriction in MVA include the occurrence of slow coronary blood flow at diagnostic angiography,29 the detection of increased circulating levels of endothelin-1 – the ‘strongest endogenous’ vasoconstrictor stimulus identified to date – both at baseline and after atrial pacing,30 and the occurrence of angina at rest as the prevailing clinical presentation. Furthermore, recent systematic assessment of intracoronary ACh testing in the cath lab in patients presenting with angina despite angiographically normal coronary arteries has shown coronary microvascular spasm in at least 25 % of these patients.19 Notably, a sizeable proportion of MVA patients has also been found to develop epicardial spasm, suggesting a possible contribution of this mechanism to the angina symptoms in at least some MVA patients.
Causes of CMVD in Primary Stable MVA
The causal mechanisms of CMVD in patients with primary stable MVA are not fully understood and are likely to be several. Traditional cardiovascular risk factors for CAD are known causes of CMVD, although there is no clear evidence of a direct relationship among risk factors and the severity of CMVD in MVA patients.31 Increased adrenergic activity and/or abnormal function of cardiac sympathetic nerve fibres have been also suggested as causal factors in some studies,32 and inflammatory mechanisms were reported to have a role in MVA.33 Also importantly, oestrogen deficiency has been advocated as a causal mechanism in women with MVA.11
Clinical Outcome
Prognosis in primary stable MVA patients showing completely normal coronary arteries has consistently been reported to be good, with rates of major cardiovascular events (i.e. death or acute myocardial infarction) that appear to be similar to those found in the general population. 9,10,34 Larger studies, however, have recently challenged the view that MVA carries a good long-term prognosis.35 These studies, however, included more heterogenous groups of patients, with potential markers of worse outcome, including subcritical coronary atherosclerosis, impaired LV function and arrhythmias. Quality of life is, on the other hand, negatively affected by MVA, with many patients needing to retire from work at an early age and restrict their social activities dramatically.
Treatment
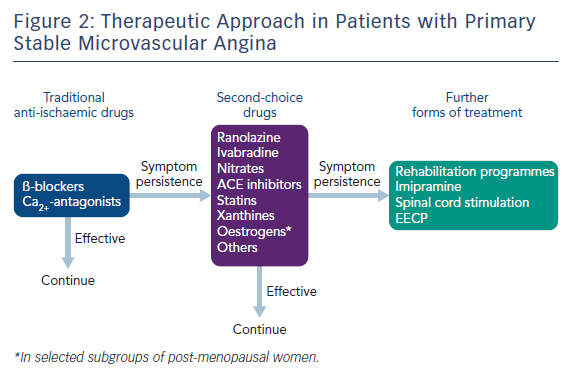
Treatment of MVA includes full control of cardiovascular risk factors and all other conditions that might impair clinical outcomes (i.e. inflammatory status, oestrogen deficiency, high adrenergic activity). Given the problematic nature of symptoms in these patients, a specific goal in the treatment of stable MVA patients is to reduce angina symptoms and improve quality of life (Figure 2). Symptomatic outcome seems favourable in primary stable MVA patients when pharmacological and non-pharmacological interventions are systematically applied. However, although a sizeable proportion of patients show a significant reduction of symptoms over time,34 some patients report worsening of angina during follow-up, which can be related to a progression of their CMVD, worsening of enhanced pain perception or exacerbation of microvascular spasm. Although conventional antianginals are considered to represent first line treatment for myocardial ischaemia and chest pain in MVA, they are not effective in many patients. A pathophysiologically based approach should be preferred over a ‘first line’ and ‘second line’ recommendation. Identifying the prevailing mechanism for MVA in every individual may help the treating physician decide whether they should try to affect myocardial oxygen demand, microvascular spam or abnormal vasodilation, or all three. Overall, beta-blockers have been shown to improve angina symptoms, particularly in MVA patients with effort-induced angina and evidence of increased adrenergic activity, e.g. high heart rate at rest and/or rapid increase of heart rate on effort.36 Ivabradine – a bradycardia-inducing drug – may be a suitable alternative option in these patients, albeit no large studies have been conducted in this clinical setting with this agent. Non-dihydropyridine calcium-channel blockers such as diltiazem, are expected to be effective in patients with angina at rest, often triggered by microvascular spasm. Oral nitrates do not appear to be effective in MVA but nicorandil, a potassium channel opener with nitrate-like actions, has been suggested in some studies to have beneficial effects in patients with MVA.37 Promising data have also been reported with the use of ranolazine,38 a recently introduced antianginal drug that acts by reducing the late sodium current in the myocardium, which results in improved diastolic relaxation during and after ischaemia.
Other pharmacological options to be considered in patients who do not respond to conventional anti-anginal treatment include xanthine derivatives, as these may favour coronary blood flow redistribution towards ischaemic areas creating an ‘anti-steal’ effect,39 ACE inhibitors, which may improve microvascular function by limiting the vasoconstrictor and pro-oxidant effects of angiotensin II,40 alpha-adrenergic blocking drugs that decrease alpha-mediated adrenergic vasoconstriction,41 statins, which may improve microvascular endothelial function through several mechanisms,42 and, in menopausal women, oestrogen replacement therapy that can correct the abnormal microvascular function caused by oestrogen deficiency.11 In patients with increased algogenic perception and persisting symptoms, pharmacological agents can be used such as imipramine and amytriptiline, which affect areas in the central nervous system that modulate pain perception.43 Furthermore xanthines, which exert an anti- algogenic effect by antagonising cardiac pain nerve fibre stimulation by adenosine, may be effective in specific cases.39 In MVA patients with anginal symptoms refractory to all currently available pharmacological agents, an improvement of symptoms and quality of life at long-term follow-up has been reported with the use of spinal cord stimulation,44 which acts both by modulating nociceptive signals and reducing microvascular ischaemia through an anti-adrenergic effect, whereas no changes in clinical status were observed in untreated controls. Enhanced external counterpulsation has been also proposed by some authors, who reported improvement of symptoms and regional ischaemia in treated patients.45
Summary and Conclusions
Microvascular angina is being increasingly recognised in clinical practice. The condition affects a larger proportion of individuals than initially thought and, although there is an increased prevalence of MVA in menopausal women, the condition is by no means one that affects women only. CMVD, presenting as abnormal coronary microvascular dilation, microvascular spasm, or both, is the pathogenic mechanism underlying MVA. Both invasive and non-invasive diagnostic tools exist in clinical practice that help identify patients affected by MVA. Prognosis has been reported to be good in patients with MVA in the absence of conditions known to be associated with increased risk. Recent large studies, however, have reported prognosis to be impaired in more heterogeneous populations of patients with angina, despite angiographically normal coronary arteries, with CMVD showing a predictive value for combined cardiovascular end-points. Management of primary MVA can be challenging but pharmacological and non-pharmacological treatments exist that help improve symptoms in a large proportion of patients affected by MVA. Further research is required in areas such as non-invasive assessment of coronary microcirculation, pain perception abnormalities and pharmacological treatments aimed to target specific pathogenic mechanisms.