Percutaneous coronary intervention (PCI) is the most commonly used revascularisation modality for obstructive coronary artery disease.1 Despite significant advances in PCI over the past 40 years, severe coronary calcification remains a challenge for successful PCI.2,3 Up to 20% of patients undergoing PCI are estimated to have moderate to severe coronary calcification.4,5
Heavily calcified lesions are difficult to dilate adequately with balloon angioplasty, even with high-pressure inflation.6 Angioplasty balloons are prone to asymmetric expansion and dogboning around the site of severe calcification, increasing the risk of coronary dissection and perforation.7,8 Calcified plaques impede delivery of angioplasty balloons and stents and increase the risk of stent underexpansion and malapposition.9,10
Vigorous advancement of drug-eluting stents (DES) across heavily calcified lesions also poses a risk of damage to the drug coating. Moreover, there might be inadequate diffusion of the drug through extensive calcium arcs to the subintima limiting the effectiveness of the DES.11 Therefore, even in the contemporary era, moderate to severe lesion calcification is associated with a higher rate of major adverse cardiovascular events (MACE), target lesion revascularisation (TLR) and target vessel revascularisation at follow-up for patients with a DES.12,13 This is likely attributable to both lesion- and patient-specific factors, since significant coronary calcification is more prevalent with advanced age, renal insufficiency, diabetes and previous coronary bypass surgery (CABG), which are independent predictors of adverse ischaemic events.5,14
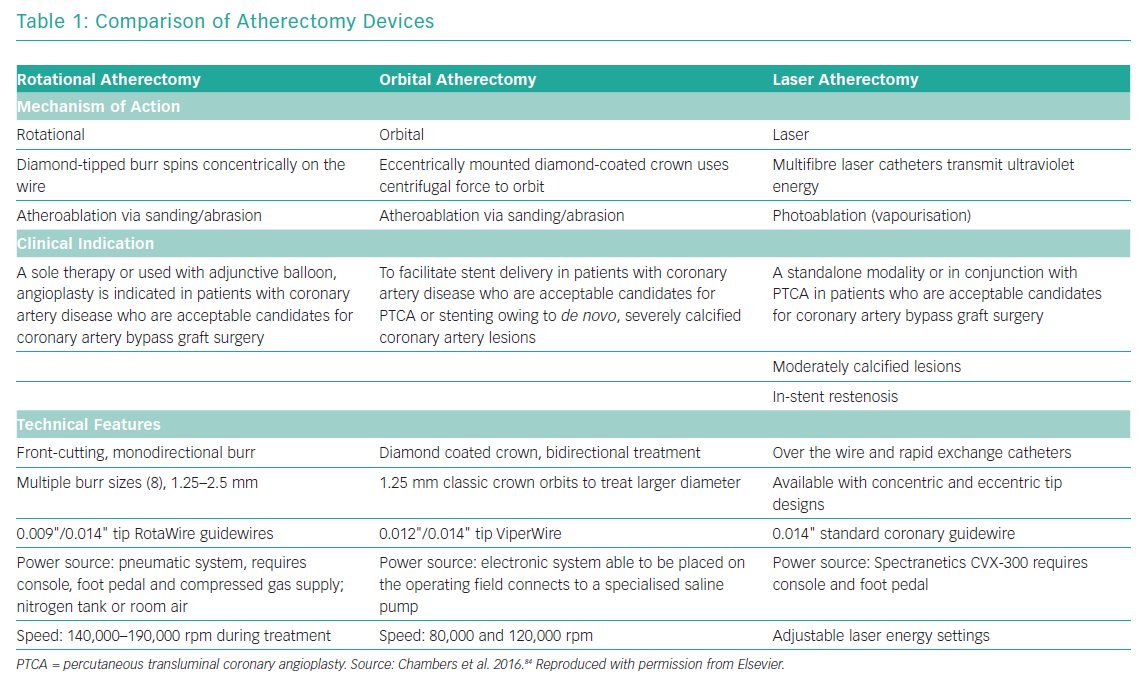
The advent of intravascular imaging has provided insights into mechanisms of stent failure and highlighted the importance of optimal lesion preparation prior to stent implantation.15 The interventional devices to modify calcified lesions before balloon angioplasty and stenting can broadly be divided into non-atherectomy and atherectomy strategies. Non-atherectomy devices, also known as modified balloons (MB; scoring, cutting, or semi-compliant constrained balloons) treat calcified plaques by cutting or targeted dissection. The atherectomy devices are aimed at physical removal of plaque material and include rotational atherectomy (RA), orbital atherectomy and excimer laser coronary angioplasty.5 Table 1 provides a brief comparison of the three atherectomy modalities. Although there have been no head-to-head randomised comparisons of RA versus orbital atherectomy, observational data suggest equivalence for the two approaches.16,17 Intravascular lithotripsy modifies plaque by causing calcium fracture and is an additional approach currently under investigation in clinical studies.18
The commercially available Rotablator (Boston Scientific) was first introduced by David Auth and colleagues in 1988.19 After initial adoption, the enthusiasm for RA was tempered after reports of high restenosis rates in the pre-DES era.6,20,21 However, with an ageing population and increase in complexity of the patient population being referred for catheterisation for PCI, there has been a resurgence of interest in RA with increased focus on optimal technique.14 The contemporary use of RA in PCI in Europe and the US varies from 1 to 3% of all PCIs performed.22 In this article, we discuss the principles, technical considerations, indications, clinical evidence and complications of the use of RA in modern PCI.
Principles
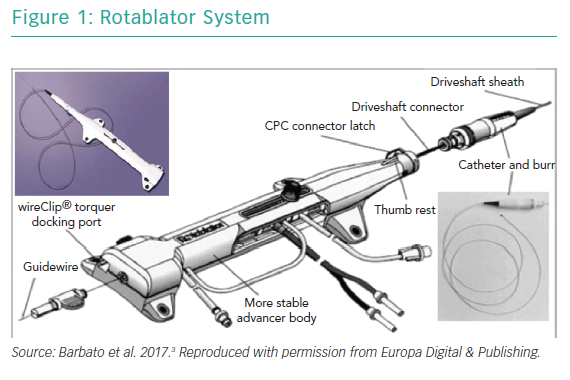
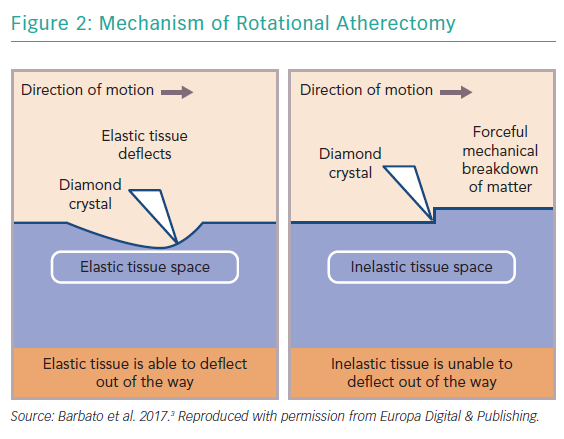
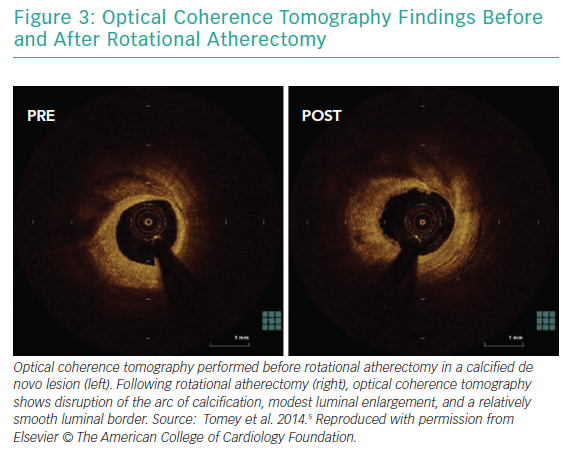
RA ablates calcified plaque using a diamond-encrusted elliptical burr, rotated at speeds of 140,000 to 180,000 rpm by a helical driveshaft, that is advanced across the lesion over a guidewire (Figure 1). The burr causes ‘differential cutting’ and preferentially ablates hard, inelastic, calcified plaque that is unable to stretch away from the RA burr compared with healthy arterial tissue (Figure 2).5 In contrast with balloon angioplasty, which produces intimal and medial dissections in calcified lesions, RA leads to lesser tissue injury and yields relatively smoother luminal surfaces and cylindrical geometry (Figure 3).23–25
The emphasis of the use of RA has shifted over the years from plaque debulking to plaque modification to facilitate balloon angioplasty and stent expansion. The transition in the approach has been driven by the results of the Study to Determine Rotablator and Transluminal Angioplasty Strategy (STRATAS) and Coronary Angioplasty and Rotablator Atherectomy Trial (CARAT) clinical trials. These trials showed that an aggressive strategy with large burrs (burr/artery ratio >0.7) to achieve maximum debulking as a lesion debulking strategy was associated with higher rates of angiographic complications, TLR and peri-procedural creatine kinase-myocardial band (CK-MB) release compared with use of smaller burrs (burr/artery ratio ≤0.7) to modify lesion compliance with a lesion modification strategy without any clinical advantages in terms of procedural success or gain in luminal diameter.26,27 This change in approach has allowed the use of smaller burrs, guide catheters and sheaths without compromising clinical efficacy.
Indications and Clinical Outcomes
Indications
The principal indication for RA is modification of severely calcified de novo coronary stenoses which are unlikely to expand adequately with balloon angioplasty to allow for complete stent expansion. The detection of severe coronary calcification traditionally relied on fluoroscopy but it has been demonstrated that fluoroscopy is less sensitive in calcium detection compared with intravascular imaging.28 Severe coronary calcification on fluoroscopy has been described as radio-opacities noted without cardiac motion before contrast injection involving both sides of the arterial wall.29
With intravascular ultrasound, severe calcification, indicated by bright echodensity causing attenuation of deeper structures, is defined as a large arc of superficial calcium involving ≥3 quadrants.28 On optical coherence tomography (OCT), areas of coronary calcification appear well demarcated and signal-poor. OCT measures of coronary calcification predictive of stent underexpansion include: length >5 mm; arc >180°; and maximum thickness >0.5 mm.30–32 RA can be used as a primary strategy in the presence of severe calcification or as a secondary approach after failure to dilate a lesion with coronary angioplasty. Although long-term outcomes are similar with planned versus bailout RA, planned RA is associated with lower procedure and fluoroscopy time, lower contrast volume and fewer in-hospital MACE.33 Therefore, if there is a strong preprocedural likelihood of the use of RA in severely calcified lesions, operators should have a low threshold for adopting a planned RA strategy. In addition, although rarely an issue, there is concern about performing RA in a lesion that has undergone aggressive balloon angioplasty because of possible unrecognised intimal dissection.
Specific Lesion Subsets
There are certain lesion-specific considerations when using RA. RA provides an effective treatment option in patients with calcified unprotected left main (ULM) disease who are not eligible for CABG. In a report of 40 patients undergoing ULM RA, there was one procedural death and 12 deaths at 2-year follow-up.34 In the ROTATE registry, although there were no differences in rates of in-hospital MACE between ULM (n=86) compared with non-ULM (n=962) RA (5.8% versus 8.0%; p=0.47), 1-year MACE was higher in the ULM group (26.4% versus 14.9%; p=0.002), largely driven by target vessel revascularisation (20.3% vs 12.7%; p=0.05). Even definite/probable stent thrombosis (ST) was higher in the ULM group (3.9% versus 0.8%; p=0.03).35 Therefore, although feasible, RA of ULM is associated with worse outcomes, likely due to the large territory of jeopardised myocardium, and a meticulous technique should be adopted.
RA is an excellent adjunct in treatment of protected LM disease in patients with previous CABG due to the high prevalence of fibrocalcific disease in this patient population.14 In patients with calcified bifurcation lesions, RA can be useful as plaques at bifurcations can be prone to plaque shift, acute side branch closure and difficult stent delivery.36 In bifurcation lesions with calcification confined to the main branch, RA of the main vessel alone is sufficient. In lesions with severely calcified, undilatable or balloon-uncrossable plaque in side branches >2.5 mm diameter, RA of the side branch should be considered.37 Care must be taken to ensure that only the RotaWire is present in the target vessel to avoid inadvertent cutting of other wires by the RA burr.
If RA of both the main branch and side branch is necessary, a novel technique has been described where after atherectomy of the main branch, the side branch is wired in a standard fashion and a guide extension advanced to the ostium of the side branch to protect the guidewire in the main branch interacting with the RA burr. The rotablator burr can then be advanced through the guide extension catheter to perform RA of the side-branch lesion. This technique does require use of larger guide catheters in order to use larger guide extensions compatible with the desired rotablator burr size.38 In chronic total occlusions, where balloons cannot be delivered after successful guidewire crossing, RA can be used for lesion modification after exchanging the crossing wire for a RotaWire over dedicated microcatheters.14 In chronic total occlusions, cases have also been described where the radiopaque portion of the RotaWire is advanced into the subintimal space or proximal cap and RA used for careful ablation of the refractory proximal cap. This technique is not routinely recommended due to the increased risk of coronary perforation.22
RA is not recommended for routine use in degenerated saphenous vein graft lesions or thrombus, although successful use in non-dilatable, calcified vein grafts lesions has been described.39 Other relative contraindications to RA include lack of onsite bypass surgery, severe three-vessel or unprotected left main disease, severe left ventricular dysfunction, lesion angulation >45° and lesion length >25 mm, although successful RA use has been reported with all these conditions.34,35,40 RA has also been successfully applied in ablation of suboptimally expanded stents. In a study of 16 patients treated with RA for severe in-stent restenosis (ISR) due to stent underexpansion resistant to balloon angioplasty, Ferri et al.41 reported an increase in minimal lumen diameter by 2.3 ± 0.8 mm and a decrease in diameter stenosis from 82.17 ± 17.2% to 11.9 ± 9.1%. There was one case of burr entrapment that was managed percutaneously. There is lack of consensus on adjunctive treatment after RA for undilatable severe ISR. In a retrospective analysis of 200 patients undergoing RA for ISR, 12-month TLR rates were lower for drug-coated balloon angioplasty (27.3%) compared with conventional balloon angioplasty (40.7%) or DES implantation (35.0%), suggesting that for severe ISR requiring a debulking strategy, drug-coated balloon angioplasty following RA might be the most effective treatment option.42 Other reports have also reported favourable results with drug-coated balloon angioplasty following RA for severe ISR.43 Nonetheless, stent ablation with RA should be used with extreme caution by highly experienced operators, ideally with on-site surgical backup.22,41
Clinical Evidence
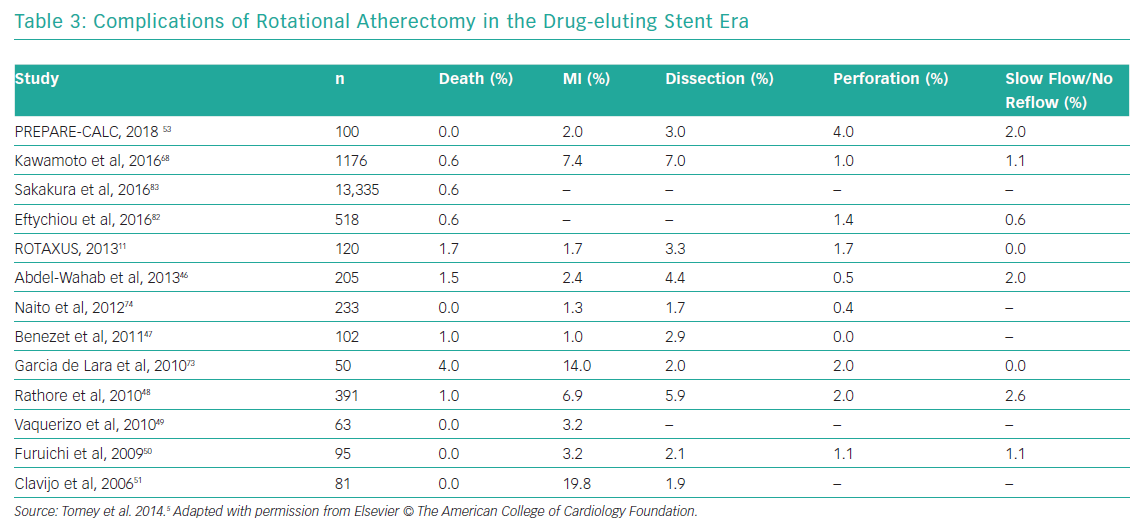
In the balloon angioplasty era, studies showed rates of restenosis with RA of approximately 40%, which is similar to using balloon angioplasty alone.20,21 During the bare-metal stent era, although RA use in calcified lesions before stenting was associated with higher procedural success with a trend towards lower restenosis rates, the absolute restenosis rates ranged between 20% and 30%.6 With the advent of DES, neointimal proliferation was significantly inhibited and the rates of restenosis and TLR were reduced in general.44,45 Several studies have reported favourable intermediate and long-term outcomes with adjunctive RA before DES implantation with TLR rates of <10% at 1- to 2-year follow-up.46–51
It is, however, unclear whether adjunctive RA in calcified lesions before DES implantation results in improved outcomes. Observational data have shown conflicting evidence and are limited by selection bias as patients undergoing RA have worse clinical and lesion-specific prognostic factors and the patients who truly benefit from RA, such as those with uncrossable lesions, are correctly not enrolled in these studies.51,52 The Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease (ROTAXUS) trial randomised 240 patients with complex calcified coronary disease in a 1:1 ratio to RA versus conventional balloon angioplasty prior to DES implantation. There was a higher strategy success in the RA group (92.5% versus 83.3%) and a higher crossover in the standard therapy group. Despite an initially higher acute lumen gain (1.56 ± 0.43 versus 1.44 ± 0.49 mm, p=0.01) with RA, in-stent late lumen loss was higher in the RA group (0.4 ± 40.58 versus 0.31 ± 0.52; p=0.04). In-stent binary restenosis, TLR, definite ST and MACE rates were similar in the two groups. The limitations of the ROTAXUS trial included missing angiographic follow-up in approximately 20% of the patients and inclusion of >50% patients with moderate coronary calcification. However, based on the ROTAXUS results, RA use for lesion preparation for calcified coronary lesions could not be routinely recommended.11 The contemporary Comparison of Strategies to PREPARE Severely CALCified Coronary Lesions (PREPARE-CALC) trial randomised 200 patients with severe calcified coronary lesions to RA versus MB (cutting or scoring balloons) angioplasty prior to implantation of third generation sirolimus eluting Orsiro (Biotronik AG) stents. Similar to ROTAXUS, strategy success was more common in the RA group (98% versus 81%). At 9 months, in-stent late lumen loss was not different between RA versus MB groups (0.22 ± 0.40 mm versus 0.16 ± 0.39 mm; p=0.21). TLR, definite/probable ST, and TVF rates were much lower than previously reported and not different between the two groups. The take home conclusions were that in modern PCI, upfront RA prior to contemporary DES in severe calcified coronary lesions was feasible and associated with greater strategy success without increases in late in-stent late lumen loss.53
Technical Considerations
Vascular Access
Considerations for vascular access should weigh the risk of bleeding and vascular complications with adequate support for performance of RA. A standard 6 Fr guiding system is sufficient for performance of RA with burr sizes up to 1.75 mm. For burr sizes ≥2.0 mm, a 7 Fr or larger system is required. Therefore, most cases requiring RA can be performed with transradial access, which is a proven strategy to mitigate bleeding complications.54 Even for larger burr sizes, transradial access is possible with use of either sheathless guiding catheters or 7 Fr slender sheaths with a 6 Fr outer diameter.14 Although the choice of guide catheters depends on the vessel anatomy and the expected need for back-up support, single curve catheters such as EBU or XB might be associated with less resistance to burr advancement to the catheter tip.22 Using larger guide catheters, deep intubation of guiding catheters, and a guide extension catheter are methods to improve back-up support during RA. A novel ‘buddy-wire’ technique has also been reported for stronger back-up support where the operators placed a supportive guidewire (Grand Slam, ASAHI INTECC) in the left anterior descending artery next to the rotablator drive shaft sheath while performing RA in a highly tortuous obtuse marginal artery. Important precautions when using the side-branch buddy-wire technique include positioning the RA burr proximal to the target lesion before wiring the side branch and activating the RA burr only distal to the side branch.55
Wiring
The RotaWire (Boston Scientific) is available in two versions, the standard Floppy or Extra Support. Both wires are 325 cm long, permitting over-the-wire exchanges and are 0.009 inches in diameter, tapering to 0.005 inches before terminating in a 0.014 inch spring tip. The distal spring tip prevents the burr from travelling beyond the tip of the wire.14 The Floppy wire is more flexible, with a longer taper (over 13 cm) and shorter 2.2 cm spring tip, causes less vessel straightening and wire bias and permits atherectomy at greater curvature of angulated lesions. The Extra Support has a shorter taper (over 5 cm) and longer spring tip (2.8 cm) compared with the floppy wire. It causes more vessel straightening and wire bias and is useful in ablation of plaque at lesser curvature of angulated lesions, aorto-ostial lesions and distal lesions. This wire is rarely used in routine clinical practice.5 Although lesions can be primarily wired with the RA wire, most experts recommend wiring with a suitable coronary guidewire and then exchanging for the RA wire over a microcatheter or over-the-wire balloon. To avoid wire fracture or burr lodging, particular care should be given to positioning the radiopaque tip of the RA wire as distal as possible from the target coronary segment. Also, it is important to loop the tip of the RA wire smoothly to prevent loops or positioning in small distal branches to avoid wire perforations and wire fracture.22
Burr Sizing, Motion and Ablation Speed
As mentioned above, the smaller burrs with burr:artery ratio <0.7 result in similar angiographic and procedural success compared with larger burrs with fewer angiographic complications, lower CK-MB release, and smaller sheath and guide catheters.26,27 A single 1.5 mm burr suffices for most vessels <3 mm in diameter and a 1.75 mm burr for vessels >3 mm in diameter. Larger burrs may be required for aorto-ostial lesions or larger vessels in which smaller sized burrs would not make physical contact with the plaque. In the presence of extreme tortuosity or angulation, long segments of severe diffuse disease, or inability to pass microcatheter across the lesion, a step-up approach with the careful use of a 1.25 mm burr with subsequent upsizing or more supportive/larger guide catheters might be required. Smaller burr sizes might also be needed in case of excessive decelerations of larger sized burrs.14,22
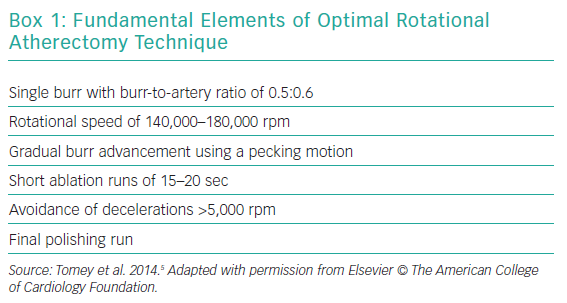
A pecking motion of the RA burr – a quick-push forward/pull-back movement – is the most widely recommended motion pattern. The following predictors of procedural complications have been described: deceleration during rotablation >5,000 rpm; motion pattern of the burr; speed of rotablation; duration of individual runs.56 The pecking motion minimises the decelerations of the RA burr and shortens the duration of individual runs (recommended <30 seconds). A recent Japanese study of 300 lesions treated with RA reported that 97 had moderate (5,000–10,000 rpm) and 21 had severe (>10,000 rpm) speed reduction. An ostial RCA lesion and total ablation time were found to be independent predictors of moderate speed reduction and an ostial RCA lesion, use of intra-aortic balloon pump, and higher systolic blood pressure before RA were predictors of a severe speed reduction.57 The ideal rotablation speed is recommended to be about 140,000 to 180,000 rpm with lower speeds being associated with burr entrapment and higher speeds linked to greater platelet activation. A recent study of 100 patients with calcified coronary artery disease randomised 1:1 to low-speed (140,000 rpm) or high-speed (190,000 rpm) RA showed no difference in incidence of slow flow (24% in both groups) or periprocedural MI (6% in both groups), suggesting that high-speed RA can be used safely with an appropriate technique when needed.58 Visual, tactile and auditory feedback provide signals of resistance to burr advancement. Once the lesion has been fully crossed, a final polishing run should be done, which should be able to be performed without resistance.14,22 Box 1 lists the key elements in optimal techniques of RA.
Pharmacology
Patients undergoing RA should receive antiplatelet and antithrombotic agents similar to standard PCI protocols. Glycoprotein IIb/IIIa that were previously shown to reduced periprocedural CK-MB release and slow-flow phenomenon, are no longer recommended routinely but can be considered on a case-by-case basis.22,59
Flush Cocktail
The side port of the rotablator advancer is connected to a pressurised flush solution that serves to lubricate device motion, mitigate heat generation and prevent sudden decelerations and flows continuously through the sheath. The traditional flush solution includes heparinised saline, vasodilators and Rotaglide lubricant, a proprietary solution containing egg whites and olive oil.22 In patients with egg allergy, elimination of the lubricant has been described with similar procedural success.60 Vasodilators have been traditionally included in the flush solution to reduce the risk of microvascular obstruction but are associated with side-effects such as bradycardia and hypotension. The drugs that have been studied include nitroglycerin, verapamil, nicardipine, adenosine, and nicorandil.61–63 There have been recent reports of effective RA eliminating the use of vasodilators while maintaining low rates of slow-flow/no-reflow.64 A recent consensus statement on RA recommends a flush solution comprised of heparinised saline and Rotaglide, reserving vasodilators for provisional use.14
Temporary Pacing
Traditionally, the use of temporary pacing during rotablation of the right coronary artery (RCA) or dominant left circumflex artery was considered routine due to concerns for transient conduction blocks with microvascular embolisation. However, with improved technique and provisional use of vasodilators such as adenosine and calcium channel blockers, most operators consider the risks with temporary pacemaker placement to outweigh the purported benefits and favour initial management with atropine (administered 0.4–1.0 mg IV every 3–5 minutes as needed) and vagolytic manoevres.14,22 IV aminophylline use (250–300 mg over 10 minutes) during RA of RCA to prevent bradyarrhythmias has also been described in small case series.65
Mechanical Circulatory Support
Use of RA is not by itself an indication for use of mechanical circulatory support. However, when used for haemodynamic indications, mechanical circulatory support can permit more extensive rotablation and more complete revascularisation. In the Prospective, Multi-center, Randomized Controlled Trial of the IMPELLA RECOVER LP 2.5 System Versus Intra Aortic Balloon Pump (IABP) In Patients Undergoing Non Emergent High Risk PCI (PROTECT II) trial, RA was used in 32 patients in the Impella group versus 20 patients in the IABP group. RA was used more aggressively with Impella (longer duration, more passes per lesion and patient, more RA in left main) resulting in higher incidence of periprocedural MI but lower rates of repeat revascularisation at 90 days.66 If transient hypotension is encountered periprocedurally during RA, IV infusion of inotropic agents such as dopamine (5–10 µg/kg/min IV) or dobutamine (2–20 µg/kg/min IV) can be considered.14
Procedure Completion and Stent Implantation
RA should be stopped when sufficient plaque modification allows optimal balloon dilation and stent implantation. At the conclusion of RA, the burr should be removed by activating the Dynaglide mode and pressing the brake defeat button. The device is then manually withdrawn while stepping on the foot pedal at low rotational speed while an assistant advances the RotaWire with an aim to maintain the wire position. Cineangiography should be done to exclude angiographic complications and the RotaWire should then be exchanged for a standard guidewire. Balloon angioplasty with an adequately sized non-compliant balloon should then be performed.
A recent OCT-based study compared the effects of calcium modification and stent extension between cutting balloon (n=18) versus conventional balloon (n=23) angioplasty following RA. Final post-stent OCT showed that the number and thickness of calcium fracture were greater after cutting versus conventional balloon, resulting in better stent expansion (78.9% versus 66.7%; p<0.01). Cutting balloon use was an independent predictor of the presence of calcium fracture and greater stent expansion, suggesting that this might be a superior lesion preparation strategy following RA prior to stent implantation.67 The presence of residual dogboning of angioplasty balloon at low-pressure inflation indicates inadequate plaque modification and merits consideration for a larger sized burr. Contemporary DES are the standard treatment of choice following RA.14,22 Analysis of the multicentre ROTATE registry showed DES used to be associated with 68% reduction in follow-up MACE after RA.68
New Iteration
In 2018, Boston Scientific launched a new generation of the Rotablator system, called the ROTAPRO, aimed at simplifying the use of RA technology.69 The foot pedal has been eliminated and replaced with a button on the top of the burr control knob, similar to the current orbital atherectomy device. The Dynaglide pedal has also been replaced by a button on the side of the advancer. The assembly is easier, more streamlined, with a new digitised display with deceleration indicators on the control console.
Complications of Rotational Atherectomy
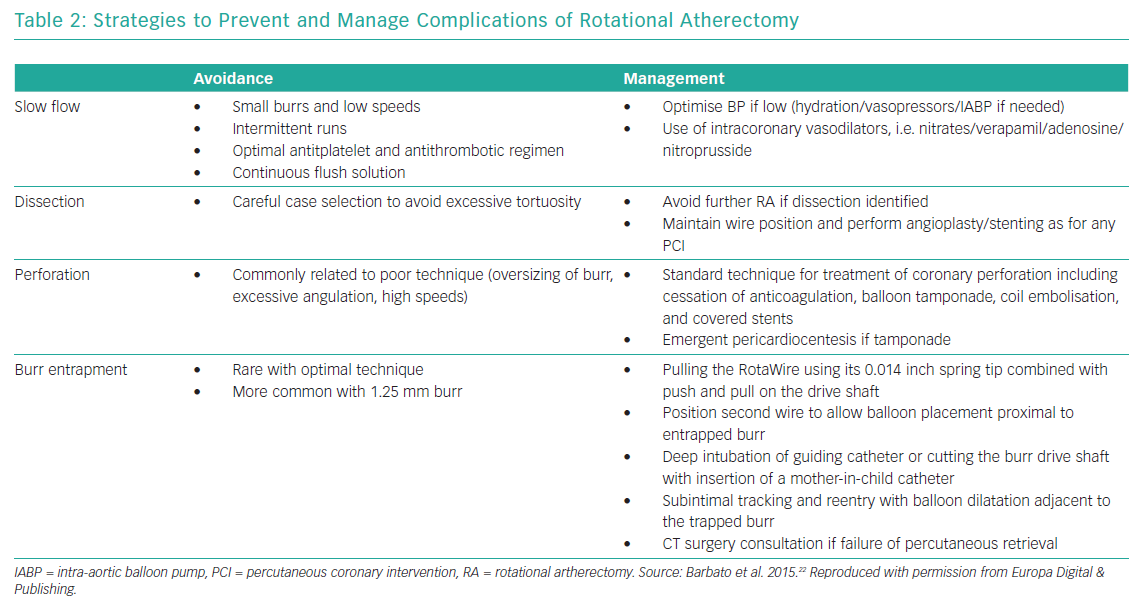
Table 2 summarises the most common complications of RA and management strategies and Table 3 reports the reported complications rates of RA in the DES era.
Slow Flow/No Reflow
Slow-flow/no-reflow results from microvascular embolisation of atherosclerotic debris and thrombi, platelet activation and vasoactive mediators and it is associated with periprocedural MI.70 It has been hypothesised that microcavitations created by the rotating RA burr across the lesion also contribute to slow flow/no reflow. This transient microcavitation phenomenon has been demonstrated in vivo by enhanced myocardial echo contrast effect and directly correlates with rotablation speed, burr size and duration.71,72 The incidence of this complication is up to about 2.5% in contemporary reports.11,46–51,68,73 Technical factors contributing to this complication include use of larger burrs, longer runs and sudden decelerations.22 Optimal technique, use of continuous flush solution, and antithrombotic therapy are preventive strategies. The cornerstone of treatment is administration of intracoronary vasodilators, ideally distally via a microcatheter. It is also critical to maintain adequate coronary perfusion pressure with hydration, vasopressors or IABP if needed.14
Dissection and Perforation
The incidence of coronary dissection with RA varies from 1.7% to 5.9% in the DES era.5,47,74 Once a severe dissection is identified, RA should be stopped, and focus should be on maintaining wire position in true lumen and completion of PCI with balloon angioplasty and stenting. If the antegrade flow is preserved, the procedure could be stopped, and the procedure reattempted in 3–4 weeks after the dissection heals.22
Coronary perforation due to rotablation is rare with reported incidence of up to 2% and it is usually related to poor technique with oversizing or forceful advancement of burr. It must, however, be noted that coronary perforation rates with PCI with RA are higher than PCI without RA (approximately 0.4%).75 In fact, a report from the British Cardiovascular Intervention Society (BCIS) database identified RA to be an independent predictor of coronary perforation (adjusted OR 2.25 [1.29–3.93]) during PCI of native vessels in patients with prior CABG.76 Lesion-specific predictors of perforation during RA include extreme tortuosity, angulation, long lesion length and location in RCA or left circumflex artery.77 Wire bias related to vessel angulation or tortuosity contributes to both dissection and perforation and can be attenuated by using the more floppy RA wire. Treatment strategies include cessation of anticoagulation, balloon tamponade, coil embolisation and covered stents. Emergent pericardiocentesis might also be required in the presence of pericardial tamponade.14
Burr Entrapment
Burr lodging within a lesion is a serious complication of RA that might require surgical intervention.78 Once stalled within a lesion, retrograde ablation is not possible because of the presence of diamond chips on the front but not the rear of the RA burr. This complication is more common with the 1.25 mm burr due to its fusiform shape which can result in sudden forward movement if the tension in the system was not removed before starting RA. Careful, conservative technique with small burr sizes, short duration of runs, short segment ablation and the pecking motion are crucial in preventing burr entrapment. Percutaneous salvage options include: pulling the RA wire using its 0.014 inch spring tip combined with push and pull on the drive shaft; balloon dilation proximal to the entrapped burr via same or different guide catheter from a different access site; deep intubation of guiding catheter or cutting the burr drive shaft with insertion of a mother-in-child catheter; and subintimal tracking and re-entry with balloon dilatation adjacent to the trapped burr.14,79–81 If retrieval is unsuccessful with the above catheter-based techniques, a cardiac surgery consultation will be necessary.
Clinical Predictors of Rotational Atherectomy Complications
Optimal RA techniques, as described above, are key to prevent periprocedural complications. A multicentre UK study identified peripheral vascular disease, diabetes, presentation with acute coronary syndromes and higher SYNTAX score to be predictors of major adverse cardiovascular events following RA.82 Similarly, a report from the Japanese PCI (J-PCI) registry of 13,335 RA cases identified older age, impaired kidney function, previous MI, emergent PCI, triple-vessel disease and lower institutional RA volume as independent predictors of worse periprocedural outcomes after RA.83
Conclusion
With increasing complexity of the patient population presenting for PCI and improved recognition of severe coronary calcification with the use of intravascular imaging, there is renewed interest in the use of atheroablative strategies for optimal lesion preparation before stent deployment. The accumulated experience in the use of RA over the last several decades has informed the current best practices in optimal technique that minimise associated complications without compromising efficacy. Despite lack of definitive evidence showing superior clinical outcomes in the contemporary DES era, randomised data have shown that RA is associated with greater procedural success in treatment of severely calcified lesions. Therefore, RA remains an integral tool for modern-day complex PCI.